Growing plants from seed can be great fun and offers the home gardener a chance to feel great satisfaction in their achievement especially when the first flower appears. Most people consider seeds easy to grow but some seeds are particularly problem some while others are almost impossible to acquire. Like all plants that produce their offspring from seed or spore the methods are similar but nature has hidden secrets to avoid using all her resources at once. Remember nature has been doing this for millions of years and has been very successful so there is no reason why you shouldn’t give it a go.
Generally speaking I believe seeds are more reliable quicker and give greater DNA variability in foliage, colour, flower size and shape. Like people we are not all the same and plants have the same variation in their own little worlds which makes them interesting.
The seed raising or potting mix must be weed free, low in salt, good moisture retention, good aeration, be light weight and have a pH of 6pH to 6.8pH unless alkaline plants are being grown in which a small quantity of lime, crushed egg shells or crushed washed oyster shells can be added.
Most seedlings and cuttings will grow well on the following mix. A good all round mix can be made by using equal parts of sand, Perlite, Vermiculite and Coir.
The coir can be replaced with peat moss for plants that originate from swamps or wet areas.
A small quantity of pine bark can be added to the mix to improve aeration and drainage if the plants are more sensitive to moisture.
Perlite: Perlite looks like white or pale grey Styrofoam. It’s a volcanic mineral that does not affect the nutrient quality or the pH of the mix. It has an excellent drainage, air and water retention property which makes it ideal as the base of soilless mixes. It is slightly acidic to neutral.
Vermiculite: Vermiculite is those silvery-grey or golden coloured shinny flecks you see in potting soil. It’s derived from heating mica to high temperatures very quickly. As it expands and explodes its water holding capacity increases dramatically. The particles soak up water and nutrients holding them in the mix until the plants are ready to access them. The aeration properties are poor so it should be used with perlite. It is slightly acidic to neutral.
Bark: Fine bark particles are added to improve drainage and air space within the mix. This means it will also decrease the water retention. Bark mixes are better for use with mature plants that need to dry between watering than for starting seeds. It is slightly acidic.
Coir: Coir is a coconut fiber by-product and works similar to peat in providing good drainage while also retaining water. It’s often used as a substitute in areas where peat is hard to come by. It is slightly acidic to neutral.
Peat: Peat has excellent moisture retention properties but can become rather soggy creating an anaerobic condition when used with seeds or cuttings that take a long time to germinate or strike. It is acidic.
Sand: Sand has poor to little moisture retention properties but has good aeration properties. It has no ability to retain nutrient essential to plant life. . It is neutral.
- Check to see what treatments are required for the various seeds you wish to sow. Collect seeds from natural habitats close to your property for best results, which are the best gene pool for your area. Buy seeds from reputable dealers and collectors or botanic gardens. Remember seeds collected from garden specimens may have been cross pollinated with other closely related species or may have genetic differences and tolerances to your district.
- Prepare the mixes. Soak the mix and remove to allow draining.
- Collect the materials and seeds to a central position. Remember the size of the seed. Seeds greater than 8mm in diameter should be sown in individual native tubes. The pre treatment of seeds that need pre treatment and those seeds that are particularly difficult to transplant should have been done earlier. Pre treatment of seeds is discussed below.
Seeds that resent transplanting are best sown in individual tubes or even peat jiffy pots so that the whole pot can be planted at a later date once the seeds have germinated.
- Place the prepared mixed into the appropriate seed tray, tubes or pots.
- Level off and tap down. The trays tubes or pots should be filled to about 80mm. Do not press down. Some seeds may need to be planted directly into the ground I have discussed this under the individual plants.
- As a general rule cover the seeds to a level equal to their diameter unless specified. That is a 5mm seed should have a covering of 5 mm of mix. Some seeds require more air flow or light so these seeds will need to be left uncovered.
- Gently water so the mix settles down.
- Place the trays in a warm, cool, sunny or shaded position depending on the plants requirements which are discussed under each species. Many shade specie prefer to be placed in a plastic bag to mimic a green house and to maintain humidity. The bag should be removed as soon as the first seedlings appear.
- Fertilise with our half strength recommended fertilizer program on a monthly basis once the seedlings have the first pair of true leaves or have been transplanted in the case of ferns. Fertilize using Seaweed, fish emulsion or organic chicken pellets soaked in water and apply the liquid on an alternate basis. Fine textured plants can have the mix applied fortnightly as a foliar spray.
- Acclimatise plants before you transplant them outdoors or into larger tubes over a 2week to 4 week period.
Seeds
There are several types of seeds. Some plants require treatment prior to sowing while others must be treated to stimulate germination prior to sowing. I have discussed the individual requirements further under plant descriptions for each variety.
Seeds: Seeds that do not require pre treatment can be sown as per step 6. When the seedlings are 25mm to 50mm tall, prick them out and plant them into 50mm native tubes using a good organic mix. Fertilize using Seaweed, fish emulsion or organic chicken pellets soaked in water on an alternate basis will help promote healthy growth.
Seeds: Because it is near impossible to transplant some plants, these seeds are best sown directly into jiffy pots or peat pots. Some smaller annuals can be planted into egg cartons. Place two or thee seeds into each carton. Once the seedlings reach 50mm to 70mm in height plant the pot and plant directly into the soil where they are required. Fertilize using seaweed, fish emulsion or organic chicken pellets soaked in water on an alternate basis will help promote healthy growth.
Seeds: Seeds that resent root disturbance need to be sown directly into the soil where the plants are required. Once the seedlings have produced two adult leaves, thin out leaving the two strongest plants. When the plants are 100mm to 120mm the smaller plant can be removed and fertilize using seaweed, fish emulsion or organic chicken pellets; if the species is not sensitive to phosphorous, soaked in water for healthy growth.
Pre Treatment of Seeds:
The pre treatment of seeds before sowing is particularly beneficial on seeds that won’t germinate quickly or germinate erratically over many weeks or even years.
- 1.Pre-treat by placing the seeds into a glass of Tepid water and allow soaking for 16 to 24 hours. This is the most common pre-treatment method used with seeds from many lilies and marsh plants in which the seeds are hard.
- Pre treat by placing the seeds into a glass of Tepid water and allow soaking for 36 to 48 hours. This is the most common pre-treatment method used with seeds from many lilies and marsh plants in which the seeds are hard.
- Pre treat by placing the seeds into a glass of very Hot Water not boiling and allow soaking for 10 to 20 hours. Seeds that soften and swell from 1.5 to 2 times their original size can be sown immediately.
- Pre treat by placing the seeds into a glass of Boiling Water and allow soaking for 10 to 24 hours. Seeds that have not responded by swelling repeat the process. This is the most common pre-treatment method used with seeds from most Senna, Acacia and all of the ‘pea’ family in which the seeds are hard.
The seeds to be treated are placed in a container, covered with boiling water and allowed to stand overnight. Seeds that soften and swell from 1.5 to 2 times their original size can be sown immediately. Any seeds which float are usually infertile and can be discarded. Those that have not swollen need to be retreated. Seed that does not swell after several repeated soaking may need to be treated differently, such as by abrasion – see below.
Scarification or abrasion SA:
This can be used as an alternative to boiling water. Seeds are gently rubbed between two sheets of fairly fine sandpaper or emery cloth to reduce the thickness of the seed coat. This can be cumbersome for large volumes of seeds and an alternative is to glue emery paper or sandpaper to the inside surfaces of a small plastic container, put the seeds in and then shake the container vigorously until the first seeds begin to display the inner cotyledon.
Some seeds only need to be nicked with a knife on one side.
Stratification STVC or STF:
When dormancy is encountered; with species native to alpine or semi-alpine habitats, a requirement for a period of cold conditioning prior to germination is required. This requirement can be replicated very easily by placing seed in a closed container; either dry or containing moist vermiculite or similar material, in a refrigerator for 4 weeks to 12 weeks prior to sowing. Various seeds will require different positions in the fridge. Most seeds will find the vegetable crisper satisfactory. Some seeds find germination more satisfactory if moved to the freezer over the last week or two. This procedure is referred to as “stratification” and examples of seed requiring this treatment are Banksia canei, Banksia saxicola, Eucalyptus kybeanensis, Eucalyptus regnans and Eucalyptus delegatensis to mention a few. Eucalyptus pauciflora benefits greatly from being placed in the freezer for the last two weeks
The method has also been applied with many grasses and lilies where the night time, winter, ground temperatures fall below zero or frosts occur. This is found in the higher altitudes to the north where frosts frequently occur, arid areas that experience black frosts and cold damp locations that may or may not freeze at night.
Fire F:
Apart from the “pea” flowers and acacias, some other seeds seem to require the passage of a bushfire to germinate. Flannel Flowers (Actinotus helianthi), for example, are usually seen at their best in the wild in the seasons immediatley following a bushfire. This effect can be simulated by sowing the seed in a terracotta pot (not plastic for obvious reasons!) and setting fire to leaf litter and twigs placed on top. The fire should be maintained for 2 to 3 minutes. This method has worked but is really a “last resort” method as it is difficult to have any real control over the amount of heat delivered to the seeds. Once the ash has cooled, the pot is watered and maintained as for any other seed raising container.
Dry Heat DH:
This method relies on placing the seeds in the oven after the evening meal has been cooked while the oven is still hot. Turn the oven off and when it has cooled to around 150 to 130 degrees centigrade place the seeds in the oven and leave them their overnight. Some specie may need the exercise repeated for 3 to 5 consecutive days (desert plants). We have found this method to be extremely successful for desert plants, arid and semi arid plants and many coastal heath plants that are subject to the periodic 7 to 15 year fire.
Smoke SM:
Research in South Africa and Western Australia has shown that smoke is a critical factor for promoting germination of seeds in areas subject to bushfires. The following articles outline some of the general principles involved:
This is a guide only as it is difficult to accurately state when a tree seed will germinate, do not discard trays too early they may germinate the following year. Some species it may be practical to sow outdoors where they will be exposed to the normal seasonal temperature fluctuations.
Ferns and Orchids are dealt with in detail below.
Tree seed sowing codes
Before you look at the codes and methods remember if in doubt as we are on some seeds it is best to apply the old adage and sow with no treatment. If the seeds are similar to another closely related species then try half your seeds using the technique of the known species.
Most moist rainforest species have the best results using fresh seeds while desert plants germinate best using older seed.
Moist rainforest plants usually require no treatment. Heath plants usually require fire or our dry heat method. Desert plants usually require boiling water, hot water, scarification or abrasion. Alpine plants usually require stratification while annuals usually require a short period of stratification or left in a dry place where the outside elements during the dry season (That is constant change of cold and warmth and respond to consistency of warmth).
Some seeds usually rainforest plants require darker cooler positions to stimulate germination while arid plants require light warmth and moisture to stimulate germination.
Overall think of the plants natural habitat before sowing.
The easiest and most difficult seeds are usually those that require nature’s interference first. Mistletoes most likely need to be partially digested that is they need to be passed through the gut quickly hence the mistletoe bird and honey eaters lend a complete hand well beak anyway.
Some seeds like the Persoonia specie may require animal interjection prior to sowing for more even constant results. Do not be disappointed if your first attempts are failures. We have all failed and still do.
NT No Treatment required.
BM The Bog method requires the trays to be standing in water at all times.
SUB Submerged
BW Boiling water to break down the seed coating.
HW Pour hot water (not boiling) over the seeds or scarify to break down the seed coating.
TW Soak in tepid or cold water for 4 to 48 hours and then sow at 20°C to 26°C.
SA Scarification or Abrasion
STVC Stratificaction Vegetable Crisper
STF Stratificaction in vegetable crisper with the final week or 2 in the freezer.
F Fire
SM Smoke
DH Dry Heat
DIV Division or Cuttings
FERN See fern spore
ORCHID See orchid seeds
Abildgaardia ovata NT
Abildgaardia vaginata NT
Abrophyllum ornans HW
Abrus precatorius HW
Abutilon oxycarpum subsp. incanum NT
Abutilon oxycarpum subsp. malvifolium NT
Abutilon oxycarpum subsp. oxycarpum NT
Abutilon oxycarpum subsp. subsagittatum.NT
Acacia – Hard Seed BW
Acacia – Soft Seed Brown seeds HW
Acacia acuminata BW
Acacia adunca BW
Acacia amblygona BW
Acacia aulacocarpa BW
Acacia baeuerlenii BW
Acacia bakeri BW
Acacia baileyana BW
Acacia beadleana BW
Acacia binervata BW
Acacia brownii HW
Acacia bruinoides BW
Acacia cangaiensis HW
Acacia chrysotricha BW
Acacia complanata BW
Acacia concurrens BW.
Acacia cultriformis BW.
Acacia dealbata HW
Acacia decora BW
Acacia disparrima BW
Acacia dunnii BW
Acacia elata HW
Acacia elongata BW
Acacia falcata BW
Acacia fimbriata BW
Acacia floribunda BW
Acacia granitica HW
Acacia hispidula BW
Acacia holosericea BW
Acacia howitti HW
Acacia implexa BW
Acacia longifolia subsp. longifolia BW
Acacia longifolia subsp. sophorae BW
Acacia longisissima BW
Acacia macrademia HW
Acacia maidenii HW
Acacia melanoxylon HW
Acacia mitchellii BW
Acacia myrtifolia BW
Acacia orites HW
Acacia oshanesii BW
Acacia penninervis BW
Acacia perangusta HW
Acacia podriifolia HW
Acacia pycnantha BW
Acacia rubida BW
Acacia ruppi BW
Acacia semilunata BW
Acacia siculiformis BW
Acacia spectabilis BW
Acacia stricta BW
Acacia suaveolens HW
Acacia terminalis BW
Acacia ulicifolia BW
Acacia venulosa BW
Acacia viscidula HW
Acacia wardellii BW
Acalypha nemorum NT
Aceratium ferrugineum NT
Acmena hemilampra NT
Acmena ingens NT
Acmena smithii NT
Acmenospermum claviforum NT
Acradenia euodiiformis NT
Acronychia acidula Careful SA
Acronychia imperforata NT
Acronychia laevis NT
Acronychia littoralis NT
Acronychia oblongifolia NT
Acronychia octandra NT
Acronychia pubescens NT
Acronychia suburosum Careful SA
Acronychia wilcoxiana NT
Acrostitchum auream FERN
Acrostichum speciosum FERN
Acrotriche aggregata NT
Acrotriche depressa NT
Actinotus helianthi SM, DH, SA, STVC
Adansonia gregorii TW
Adenanthera pavonina TW
Adiantum atheopicum FERN
Adiantum atroviride FERN
Adiantum formosum FERN
Adiantum hispidulum FERN
Aegiceras corniculatum NT
Agathis robusta NT
Agathis altropurpurea NT
Agiortia cicatricata NT
Agiortia pedicellata NT
Agiortia pleiosperma NT
Aglaia brassii NT
Agonis flexuosa NT
Aidia racemosa NT
Ailanthus triphysa
Ajuga australis NT
Akania bidwillii NT
Alangium villosum subsp. polyosmoides NT
Alangium villosum subsp. villosum NT
Albizia lebbeck BW
Alchornea ilicifolia NT
Alectryon coriaceus NT
Alectryon subcinereus NT
Alectryon tomentosus NT
Aleurites moluccana NT
Allocasuarina defungens SA
Allocasuarina littoralis SA
Allocasuarina rigida sub sp.rigida SA
Allocasuarina torulosa SA
Alloteropsis semialata NT
Alloxylon flammeum NT
Alloxylon pinnatum NT
Alocasia brisbanensis NT
Alphitonia excelsa SA
Alpinia arundelliana NT
Alpinia caerulea NT
Alstonia actinophylla NT
Alstonia scholaris NT
Alstonia spectabilis NT
Alyxia ruscifolia HW
Amyema bifurcatum NT
Amyema cambagei NT
Amyema congener subsp. congener NT
Amyema congener subsp. divergens NT
Amyema congener subsp. rotundifolia NT
Amyema gaudichaudii NT
Amyema miquelii NT
Amyema pendulum subsp. longifolia NT
Amyema pendulum subsp. pendulum NT
Amyema quandang subsp. bancroftii NT
Amyema quandang subsp. quandang NT
Amylotheca dictyophleba NT
Aneilema acuminatum NT
Angiopteris evecta FERN
Angophora costata NT
Angophora floribunda NT, STVC
Angophora paludosa NT
Angophora robur NT, STVC
Angophora subvelutina NT, STVC
Angophora woodsiana NT
Anigozanthos flavida STVC
Anigozanthos manglesii STVC
Anopterus macleayanus FERN
Antidesma bunius NT
Antidesma parvifolium NT
Aotus ericoides BW
Aotus lanigera BW
Aotus subglauca subsp. filiformis BW
Aotus subglauca subsp. subglauca BW
Aphananthe philippinensis NT
Aphanopetalum resinosum NT
Apium prostratum var. filiforme SUB
Aponogeton elongatus NT
Arachniodes aristata FERN
Araucaria bidwillii NT
Araucaria cunninghamii NT
Archidendron grandiflorum HW
Archidendron hendersonii HW
Archirhodomyrtus beckleri NT
Archontophoenix Alexandra STVC
Archontophoenix cunninghamiana STVC
Archontophoenix maxima SA
Archontophoenix purpurea SA
Arenga australasica SA
Argophyllum nullumense NT
Argyrodendron actinophyllum sub sp.diversifolia NT
Argyrodendron peralatum NT
Aristida acuta NT
Aristida benthamii sub sp. benthamii NT
Aristida calycina sub sp. calycina NT
Aristida lignose NT
Aristida queenslandica sub sp. queenslandica NT
Aristida vagans NT
Aristida warburgii NT
Aristotelia australasica NT
Arthraxon hispidus NT
Arthrochilus prolixus ORCHID
Arthropteris beckleri FERN
Arthropteris tenella FERN
Arytera divaricata NT
Asparagus racemosus NT
Asplenium attenuatum sub sp. attenuatum FERN
Asplenium australisicum FERN
Asplenium bulbiferum FERN
Asplenium difforme FERN
Asplenium flabellifolium FERN
Asplenium flaccidum sub sp. flaccidum FERN
Asplenium polyodon FERN
Asplenium nidus FERN
/span>Asterolasia correifolia NT
Asteromyrtus brassii NT
Astroloma pinifolium NT
Astrotricha cordata NT
Astrotricha latifolia NT
Astrotricha longifolia NT
Atalaya salicifolia NT
Atractocarpus benthamianus subsp. benthamianus NT
Atractocarpus benthamianussubsp. glaber NT
Atractocarpus fitzalanii NT
Atractocarpus sessilis NT
Atriplex nummularia STVC
Atriplex semibacatta STVC
Auranticarpa rhombifolium NT
Australina pusilla NT
Austrobuxus swainii NT
Austrocynoglossum latifolium NT
Austromuellera trinervia NT
Austromyrtus dulcis NT
Austromyrtus tenuifolia NT
Austrosteenisia blackii var. blackii NT
Austrosteenisia glabristyla NT
Austrostipa pubescens NT
Austrostipa ramosissima NT
Austrostipa rudis sub sp. nervosa NT
Austrostipa scabra sub sp.falcata, sub sp.scabra NT
Austrostipa scabra sub sp.falcata, sub sp.scabra NT
Avicennia marina sub sp. australisica BM
Azolla filiculoides DIV BM
Azolla pinnata DIV, BM
Backhousia angustifolia NT
Backhousia citriodora NT
Backhousia myrtifolia NT
Backhousia sciadophora NT
Baeckea diosmifolia NT
Baeckea frutescens NT
Baeckea imbricata NT
Baeckea linifolia NT
Baekea utilis NT
Baloghia inophylla NT
Baloghia marmorata NT
Baloskion pallens NT-BM
Baloskion stenocoleum NT-BM
Baloskion tenuiculme NT-BM
Baloskion tetraphyllum subsp. meiostachyum NT-BM
Baloskion tetraphyllum subsp. tetraphyllum NT-BM
Bambusa arnemica NT, DIV
Banksia aemulum NT
Banksia baxteri NT, STVC
Banksia blechnifolia NT, STVC
Banksia ericifolia sub sp. ericifolia NT
Banksia ericifolia subsp. macrantha NT
Banksia formosa NT
Banksia fraseri subsp. ashby NT
Banksia fraseri subsp. creba NT
Banksia fraseri subsp. effuse NT
Banksia fraseri subsp. fraseri NT
Banksia fraseri subsp. oxycedra NT
Banksia ilicifolia NT
Banksia integrifolia sub sp. integrifolia NT
Banksia integrifolia subsp. monticola NT
Banksia marginata NT
Banksia media NT
Banksia oblongifolia NT
Banksia paludosa NT, STVC
Banksia petiolaris NT
Banksia plagiocarpa HW
Banksia praemorsa NT, STVC
Banksia repens NT, STVC
Banksia robur NT- BM
Banksia serrata NT
Banksia spinulosa subsp. spinulosa NT
Banksia spinulosa subsp. collina NT
Banksia tenuis NT
Banksia undata NT
Barringtonia acutangula NT
Barringtonia asiatica NT
Barringtonia calyptrata NT
Bauera capitata NT
Bauera rubioides NT
Bauhinia hookeri HW
Bauhinia malabarica HW
Bauhinia syringifolia HW
Baumea acuta HW
Baumea arthrophylla HW
Baumea articulata HW
Baumea gunnii HW
Baumea juncea HW-BM
Baumea muelleri HW
Baumea nuda HW
Baumea rubiginosa HW
Baumea teretifolia HW
Beaufortia incana NT
Beilschmiedia elliptica NT
Beilschmiedia obtusifolia NT
Belvisia mucronata sub sp. mucronata FERN
Benthamina alyxifolia NT
Berberidopsis beckleri NT
Bertya brownii NT
Bertya sp. Chambigne NT
Bertya sp. Clouds Creek NT
Beyeria lasiocarpa NT
Billardiera heterophylla NT
Billardiera rubens NT
Billardiera scandens NT
Blandfordia grandiflora NT
Blandfordia nobilis NT
Blechnum camfieldii FERN
Blechnum cartilaginium FERN
Blechnum indicum FERN
Blechnum minus FERN
Blechnum nudum FERN
Blechnum patersonii subsp. patersonii FERN
Blechnum patersonii subsp. queenslandica FERN
Blechnum wattsii FERN
Blumea mollis STVC
Boehmeria macrophylla NT
Bolboschoenus fluviatilis NT
Bombax ceiba subsp. leiocarpum SA
Boronia chartacea DH
Boronia clavata DH
Boronia denticulata DH
Boronia falcifolia DH
Boronia hapalophylla DH
Boronia heterophylla DH
Boronia ledifolia DH
Boronia parviflora DH
Boronia pinnata DH
Boronia polygalifolia DH
Boronia rosmarinifolia DH
Boronia safrolifera DH
Boronia umbellata DH
Bosistoa floydii NT
Bosistoa pentacocca NT
Bossiaea ensata NT
Bossiaea heterophylla NT
Bossiaea rhombifolia subsp. concolor NT
Bossiaea rhombifolia subsp. rhombifolia NT
Bothriochloa decipiens subsp. decipiens NT
Botrychium australe FERN
Brachychiton aceriifolius NT
Brachychiton bidwillii NT
Brachychiton discolor NT
Brachychiton populneus NT
Brachychiton rupestris NT
Brachyloma daphnoides subsp. daphnoides NT
Brachyloma daphnoides subsp. glabrum NT
Brachyloma daphnoides subsp. pubescens NT
Brachyloma saxicola NT
Brachyloma scortechinii NT
Brachyscome ciliaris subsp. brachyglossa NT
Brachyscome ciliaris subsp. latifolia NT
Brachyscome ciliaris subsp. lanigunosa NT
Brachyscome ciliaris subsp. subintegrifolia NT
Brachyscome iberidifolia NT
Brachyscome microcarpa NT
Brachyscome stuartii NT
Brasenia schreberi DIV
Breynia oblongifolia STVC
Bridelia exaltata NT
Bruguiera gymnorhiza NT
Brunoniella australis STVC
Buchnera gracilis NT
Buckinghamia cellsissima NT
Bulbine bulbosa STVC
Bulbophyllum argyropus ORCHID
Bulbophyllum bracteatum ORCHID
Bulbophyllum elisae ORCHID
Bulbophyllum exiguum ORCHID
Bulbophyllum minutissimum ORCHID
Bulbophyllum schillerianum ORCHID
Bulbophyllum shepherdii ORCHID
Bulbophyllum weinthalii ORCHID
Bulbostylis barbata ORCHID
Bulbostylis densa ORCHID
Burchardia umbellata NT
Burmannia disticha NT
Bursaria spinosa NT
Byblis filifolia BM
Byblis gigantea BM
Caelospermum paniculatum NT
Caesalpinia subtropica NT
Caesia parviflora subsp. minor HW
Caesia parviflora subsp. parviflora HW
Caesia parviflora subsp. vittata HW
Cajanus pubescens SA
Caladenia alata ORCHID
Caladenia carnea ORCHID
Caladenia catenata ORCHID
Calamus muelleri NT
Calandrinia pickeringii
Calanthe triplicata ORCHID
Caldcluvia paniculosa NT
Caleana major ORCHID
Callerya australis NT
Callerya megaspermum NT
Callicarpa brevistyla NT
Callicarpa pedunculata NT
Callicoma serratifolia NT
Callitriche muelleri
Callitris baileyii SAS
Callitris columellaris SA
Callitris enderlicheri SAS
Callitris glaucophyla SA
Callitris macleayana SA
Callitris monticola SA
Callitris oblonga NT
Callitris rhomboidea SA
Calochilus campestris ORCHID
Calochilus gracillimus ORCHID
Calochilus grandiflorus ORCHID
Calochilus paludosus ORCHID
Calochilus robertsonii ORCHID
Calochlaena dubia S.
Calomeria amaranthoides NT
Calotis cuneifolia S,G.
Calotis lappulacea S,G.
Calystegia marginata NT
Calystegia soldanella NT
Calytrix tetragona NT
Canarium australasicum NT
Canavalia rosea SA
Capillipedium spicigerum NT
Capillipedium parviflorum NT
Capparis arborea NT
Cardamine microthrix NT-BM
Carex appressa TW
Carex breviculmis NT
Carex brunnea NT
Carex declinata NT
Carex fascicularis NT
Carex gaudichaudiana NT
Carex hubbardii NT
Carex inverse NT
Carex longebrachiata NT
Carex maculata NT
Carex polyantha NT
Carex pumila NT
Carpentaria accuminata NT
Carpobrotus glaucescens NT
Carpobrotus virescens NT
Carronia multisepalea
Cassia brewsteri HW
Cassia marksiana HW
Cassia tomentella HW
Cassinia compacta NT
Cassinia quinquefaria NT
Cassinia subtropicaNT
Cassytha pubescens NT
Cassytha racemosa NT
Castanospermum australe NT
Castanospora alphandii NT
Casuarina cuninghamiana HW
Casuarina equistifolia sub sp. incana. SA
Casuarina glauca SA
Caustis blakei NT
Caustis flexuosa NT
Caustis recurvata subsp. hirsuta NT
Caustis recurvata subsp. recurvata NT
Cayratia clematidea NT
Cayratia eurynema NT
Celastrus australis NT
Celastrus subspicata NT
Celtis paniculata NT
Cenchrus caliculatus NT
Cenchrus robustus NT
Centella asiatica DIV
Centrolepis fascicularis NT
Cephalaralia cephalobotrys BM
Cephalomanes caudatum NT
Cephalotus follicularis NT, TW
Ceratopetalum apetalum NT
Ceratopetalum gummiferum NT
Ceratophyllum demersum SUB
Cerbera floribunda NT
Cerbera manghas NT
Cestichis reflexa NT
Cestichis swenssonii NT
Chamaecrista maritima BM
Chamaecrista nomame HW
Chamelaucium uncinatum NT
Cheilanthes distans FERN
Cheilanthes sieberi subsp. sieberi FERN
Cheilanthes sieberi subsp. pseudovellea FERN
Chiloglottis diphylla ORCHID
Chiloglottis sylvestris ORCHID
Chiloglottis trilabra ORCHID
Chloanthes parviflora NT
Chloanthes stoechadis NT
Choricarpia leptopetala
Chloris ventricosa
Chorizandra cymbaria SA
Chorizandra sphaerocephala SA
Chorizema parviflorum BW
Christella hispidula FERN
Chrysocephalum apiculatum NT
Chrysopogon filipes NT.
Cinnamomum oliveri NT
Cinnamomum virens NT
Cissus antartica S,G. NT
Cissus hypoglauca NT
Cissus sterculiifolia NT
Citronella moorei NT
Citrus australasica NT
Cladium procerum NT
Claoxylon australe NT
Cleistanthus cunninghamii NT
Clematicissus opaca NT
Clematis aristata NT
Clematis glycinoides NT
Clematis microphylla NT
Clerodendrum floribundum NT
Clerodendrum inerme NT
Clerodendron tomentosa NT
Cochlospermum fraseri SA
Cochlospermum gillivraei SA
Cocos nucifera SA
Codonocarpus attenuatus NT
Coleocarya gracilis NT
Comesperma defoliatum NT-STVC
Comesperma ericinum NT-STVC
Commelina cyanea DIV
Commelina ensifolia DIV
Commersonia bartramia STVC
Commersonia breviseta STVC
Commersonia fraseri STVC
Conospermum taxifolium NT
Cordyline cannifolia SA
Cordyline congesta SA
Cordyline fruticosa SA
Cordyline murchinsonae SA
Cordyline petiolaris SA
Cordyline rubra SA
Cordyline stricta SA
Coronidium elatum STVC
Coronidium lanuginosum STV
Coronidium oxylepis STVC
Correa baeuerlenii STVC
Correa lawrenceana STVC
Correa reflexa STVC
Corybas aconitiflorus ORCHID
Corybas barbarae ORCHID
Corybas fordhamii ORCHID
Corybas hispidus ORCHID
Corybas undulatus ORCHID
Corymbia citriodora NT
Corymbia curtisii NT
Corymbia ficifolia NT
Corymbia gummifera NT
Corymbia henryi NT
Corymbia intermedia NT
Corymbia maculata NT
Corymbia ptycocarpa NT
Corymbia tessellaris NT
Corymbia torelliana NT
Corymbia trachyphloia NT
Corymbia variegata NT
Corynocarpus rupestris NT
Costus potierea NT
Craspedia canens STF
Craspedia variabilis STF
Crassula sieberiana STVC
Crinum pedunculatum SA
Crinum uniflorum SA
Crotalaria brevis HW
Crotalaria medicaginea subsp. linearis HW
Crotalaria medicaginea subsp. mediciaginea HW
Crotalaria medicaginea subsp. neglecta HW
Crotalaria mitchellii subsp. laevis HW
Crotalaria mitchellii subsp. mitchellii HW
Crotalaria montana subsp. angustifolia HW
Crotalaria montana subsp. Montana HW
Crotalaria verrucosa HW
Croton insularis NT
Croton stigmatosus NT
Croton verreauxii NT
Crowea exalata NT
Cryptandra longistaminea NT
Cryptandra propinqua NT
Cryptocarya bidwillii SA
Cryptocaya dorrigoensis SA
Cryptocarya erythroxylon SA
Cryptocarya floydii SA
Cryptocarya foveolata SA
Cryptocarya glaucescens SA
Cryptocarya laevigata SA
Cryptocarya meisneriana SA
Cryptocarya microneura SA
Cryptocaya nova-anglica SA
Cryptocarya obovata SA
Cryptocarya rigida SA
Cryptocarya triplinervis sub sp. pubens SA
Cryptocarya triplinervis sub sp. triplinervis SA
Cryptocarya willwilliana SA
Cryptostylis erecta ORCHID
Cryptostylis hunteriana ORCHID
Cryptostylis subulata ORCHID
Cupaniopsis anachardioides NT
Cupaniopsis baileyana NT
Cupaniopsis flagelliformis subsp. australis NT
Cupaniopsis newmanii NT
Cupaniopsis parvifolia NT
Curculigo capitata DIV,STVC
Curculigo ensifolia NT, STVC
Curcuma australasica NT
Cuscuta australis NT
Cuttsia viburnea NT
Cyathea australis FERN
Cyathea baileyana FERN
Cyathea cooperi FERN
Cyathea cunninghamii FERN
Cyathea leichardtiana FERN
Cyathea rebeccae FERN
Cyathea robertsiana FERN
Cyathea woollsiana FERN
Cycas media SA
Cyclophyllum longipetalum FERN
Cyclosorus interruptus FERN
Cymbidium canaliculatum ORCHID
Cymbidium madidum ORCHID
Cymbidium sauve ORCHID
Cymbopogon refractus NT
Cynanchum carnosum NT
Cynometra iripa NT-BM
Cynodon dactylon NT
Cyperus brevifolius STVC
Cyperus cyperoides STVC
Cyperus difformis STVC
Cyperus disjunctus STVC
Cyperus eglobosus SA
Cyperus enervis STVC
Cyperus exaltatus STVC
Cyperus flaccidus STVC
Cyperus flavidus STVC
Cyperus fulvus STVC
Cyperus gracilis STVC
Cyperus haspan STVC
Cyperus imbecillis STVC
Cyperus laevigatus STVC
Cyperus laevis STVC
Cyperus leiocaulon STVC
Cyperus lucidus STVC
Cyperus mirus STVC
Cyperus pilosus STVC
Cyperus platystylis STVC
Cyperus polystachyos STVC
Cyperus procerus STVC
Cyperus sanguinolentus STVC
Cyperus sphaeroideus STVC
Cyperus stradbrokensis STVC
Cyperus subulatus STVC
Cyperus tetraphyllus STVC
Cyperus trinervis STVC
Cyperus unioloidesSTVC
Damasonium minus NT
Dampiera diversifolia NT
Dampiera purpurea NT-BM
Dampiera stricta NT-BM
Dampiera sylvestris NT-BM
Daphnandra apatelaNT
Daphnandra micrantha NT
Daphnandra tenuipes NT
Darlingia darlingiana NT
Darwinia citriodora NT
Darwinia collina NT
Darwinia oldfieldii NT
Daucus glochidiatus NT-BM
Davallia solida sub sp. pyxidata, sub sp. solida. FERN
Davidsonia jerseyana NT
Davidsonia pruriens NT
Daviesia arborea SA
Daviesia genistifolia SA
Daviesia latifolia SA
Daviesia nova-anglica SA
Daviesia ulcifolia SA
Daviesia umbellate SA
Daviesia villifera SA
Daviesia wyattiana SA
Dawsonia superba var. pulchra NT
Decaspermum humile NT
Deeringia amaranthoides NT
Deeringia arborescens NT
Dendrobium aemulum ORCHID
Dendrobium dolichophyllum ORCHID
Dendrobium fairfaxii ORCHID
Dendrobium gracilicaule ORCHID
Dendrobium linguiforme ORCHID
Dendrobium melaleucaphilum ORCHID
Dendrobium monophyllum ORCHID
Dendrobium mortii ORCHID
Dendrobium phalaenopsis ORCHID
Dendrobium pugoiniforme ORCHID
Dendrobium schoeninum ORCHID
Dendrobium teretifolium ORCHID
Dendrobium tetragonum ORCHID
Dendrocnide excela NT
Dendrocnide moroides NT
Dendrocnide photinophylla NT
Dendrophthoe vitellina NT
Denhamia celastroides NT
Denhamia moorei NT
Denhamia pittosporoides NT
Dennstaedtia davallioides FERN
Deparia petersenii FERN
Derris involuta NT
Derris trifoliata NT
Desmodium brachypodum HW
Desmodium gunnii NT
Desmodium heterocarpon NT
Desmodium nemorosum NT
Desmodium rhytidophyllum NT
Desmodium varians NT
Deyeuxia parviseta NT
Deyeuxia reflexa STF
Dianella atrax H,L,F, S,G.
Dianella caerulea subsp. assera STVC
Dianella caerulea subsp. caerulea STVC
Dianella caerulea subsp. cinerascens STVC
Dianella caerulea subsp. petasmatodes STVC
Dianella caerulea subsp. producta STVC
Dianella caerulea subsp. protensa STVC
Dianella caerulea subsp. vannata STVC
Dianella congesta STVC
Dianella crinoides STVC
Dianella longifolia subsp. longifolia STVC
Dianella longifolia subsp. stenophylla STVC
Dianella pavopennacea STVC
Dianella revoluta STVC
Dianella tasmanica STVC
Dichanthium tenue STVC
Dichelachne crinita SA
Dichelachne micrantha NT
Dichondra repens DIV
Dicksonia antarctica FERN
Dicksonia youngiae FERN
Dictymia brownii FERN
Digitaria didactyla NT
Digitaria diffusa NT
Digitaria longiflora NT
Digitaria ramularis NT
Dillenia alata NT
Dillwynia glaberrima SA
Dillwynia phylicoides SA
Dillwynia retorta SA
Dimorphocalyx australiensis SA, NT
Dinosperma erythrococcum SA
Dioscorea transversa NT
Diospyros australis NT
Diospyros calycantha NT
Diospyros fasciculosa NT
Diospyros pentamera NT
Diplachne fusca NT
Diplazium assimile NT
Diplazium australe NT
Diplazium dilatatum NT
Diplocyclos palmatus NT
Diploglottis bernieana NT, SA
Diploglottis cuninghamii NT
Diploglottis obovata NT
Diplolaena grandiflora NT
Dipodium atropurpureum NT
Dipodium punctatum NT
Dipodium variegatum NT
Dischidia nummularia NT
Diuris chrysantha STVC ORCHID
Diuris punctata sub sp. punctata STVC ORCHID
Diuris sulphurea STVC ORCHID
Dockrillia cucumerina ORCHID
Dockrillia pugioniforme ORCHID
Dockrillia teretifolium ORCHID
Dodonaea boroniifolia STVC/SA
Dodonaea hirsuta STVC/SA
Dodonaea multijuga STVC/SA
Dodonaea rhombifolia STVC/SA
Dodonaea triquetra STVC
Dodonaea viscosa subsp. angustifolia STVC/SA
Dodonaea viscosa subsp. burmanniana STVC/SA
Dodonaea viscosa subsp. cuneata STVC/SA
Dodonaea viscosa subsp. spatulata STVC/SA
Dodonaea viscosa subsp. viscose STVC/SA
Doodia aspera FERN
Doodia australis FERN
Doodia caudata FERN
Doodia heterophylla FERN
Doodia linearis FERN
Doryanthes excelsa STVC/SA
Doryanthes palmeri STVC/SA
Doryphora sassafras NT
Dracophyllum secundum STVC
Drosera adelea BM
Drosera binata BM
Drosera burmanni BM
Drosera peltata BM
Drosera pygmaea BM
Drosera spatulata BM
Drynaria quercifolia FERN
Drynaria rigidula FERN
Drynaria sparsisora FERN
Drymophila moorei STVC
Drypetes deplanchei NT
Dubosia myoporoides STVC
Durabaculum undulatum ORCHID
Durringtonia paludosa NT
Dysoxylum fraserianum NT
Dysoxylum mollissimum NT
Dysoxylum rufum NT
Echinochloa colona BM
Echinochloa telmatophila SUB
Echinopogon caespitosus. SUB
Echinopogon intermedius SUB
Echinopogon ovatus SUB
Echinostephia aculeataNT
Eclipta platyglossaNT
Eclipta prostrataNT
Ehretia acuminata SA
756 Einadia hastata NT
757 Einadia nutans sub sp. linifolia NT
Einadia nutans subsp. nutans NT
758 Einadia trigonos sub sp. stellulata NT
759 Eleocarpus angustifolius SA
760 Elaeocarpus eumundi SA
762 Elaeocarpus holopetalus STF
763 Elaeocarpus kirtonii SA
764 Elaeocarpus obovatus SA
764 Elaeocarpus reticulatus SA
766 Elaeodendron australe SA
767 Elatine gratioloides SUB
769 Elatostema reticulatum NT
770 Elatostema stipitatum NT
771 Elattostachys nervosa STVC
772 Elattostachys xylocarpa STVC
773 Eleocharis acuta NT
774 Eleocharis cylindrostachys NT
775 Eleocharis dietrichiana NT
775a Eleocharis dulcis NT
776 Eleocharis equisetina NT
777 Eleocharis geniculata NT
778 Eleocharis philippinensis NT
779 Eleocharis pusilla BM
778 Eleocharis sphacelata NT
779 Eleocharis tetraquetra TN
780 Elionurus citreus NT
781 Embelia australiana NT
781a Emilia sonchifolia NT
782 Emmenosperma alphitonioides NT
784 Empodisma minus NT
784a Empusa habenarina NTN
785 Enchylaena tomentosa NNT
786 Endiandra compressa N NT
787 Endiandra crassiflora NT
788 Endiandra discolor NT
789 Endiandra floydii NT
790 Endiandra muelleri subsp. muelleri NT
791 Endiandra muelleri subsp. bracteata NT
792 Endiandra pubens NT
793 Endiandra sieberi NTN
794 Endiandra virens NTT
795 Entada rheedii NT
796 Enteropogon unispiceus NTEntolasia marginata NTNEntolasia stricta NTEntolasia whiteana NTEnydra fluctuans NTEpacris breviflora STVCVEpacris impressa STVCEpacris longiflora STVCEpacris microphylla STVCEpacris obtusifolia STVC/SAEpacris pulchella SA/STVCEpacris rhombifolia STVCEpaltes australis STVCEpipogium roseum NTEragrostis brownie NTEragrostis elongata NTEragrostis interrupta NTEragrostis leptostachya NTEragrostis parviflora NTEragrostis pubescens NTEragrostis sororia NTEragrostis spartinoides NTEragrostis trachycarpa NTEremochloa bimaculata NTEremophila bignoniiflora NTEremophila brevifolia NTEremophila debilis NTEremophila duttoni NTEremophila forrestii NTEremophila glabra NTEremophila longifolia NTEremophila maculata NTEremophila nivea NTEremophila sp. NTEriachne glabrata NTNEriachne pallescens NTEriachne rara NNTEriocaulon australe NTEriocaulon scariosum NTEriochilus cucullatus NTEriochloa procera NTEriostemon australasius NTEryngium expansum NTErythrina numerosa SAErythrina vespertilio SAErythrophleum chlorostachys NTErythrorchis cassythoides ORCHIDEucalyptus acaciiformis NTEucalyptus acmenoides NTEucalyptus aglomerata NTEucalyptus amplifolia NTEucalyptus angustissima NTEucalyptus baileyana NTEucalyptus bancroftii NTEucalyptus biturbinata NTEucalyptus brassiana NTEucalyptus bridgesiana NTEucalyptus brunnea STVCEucalyptus caesia NTEucalyptus caliginosa STVCEucalyptus camaldulensis NTEucalyptus cameronii STVCEucalyptus campanulata STVCEucalyptus carnea STVCEucalyptus cerasiformis NTEucalyptus crebra NTEucalyptus dorrigoensis STVCEucalyptus dunnii NTEucalyptus eugenioides NTEucalyptus fibrosa NTEucalyptus fusiformis NTEucalyptus glaucina NTEucalyptus globoidea NTEucalyptus globulus STVCEucalyptus goniantha NTEucalyptus grandis NTEucalyptus henryi NTEucalyptus kruseana NT
Eucalyptus laevopinea NT
Eucalyptus lansdowneana NT
Eucalyptus ligustrina NT
Eucalyptus marginata NT
Eucalyptus melanophloia STVC
Eucalyptus melliodora NT
Eucalyptus microcorys NT
Eucalyptus moluccana NT
Eucalyptus nobilis STVC
Eucalyptus notabilis NT
Eucalyptus obliqua NT
Eucalyptus ophitica NT
Eucalyptus oreades NT
Eucalyptus pachycalyx NT
Eucalyptus pachyphylla NT
Eucalyptus phoenicea NT
Eucalyptus pilularis NT
Eucalyptus planchoniana NT
Eucalyptus platyphylla NT
Eucalyptus pleurocarpa NT
Eucalyptus prava STVC
Eucalyptus propinqua NT
Eucalyptus psammitica NT
Eucalyptus pyriformis NT
Eucalyptus pyrocarpa NT
Eucalyptus quadrangulata NT
Eucalyptus resinifera NT
Eucalyptus robusta NT
Eucalyptus rummeryi NT
Eucalyptus saligna NT
Eucalyptus seeana NT
Eucalyptus sepulcralis STVC
Eucalyptus siderophloia NT
Eucalyptus sideroxylon NT
Eucalyptus signata NT
Eucalyptus sp. 1 STCF
Eucalyptus sp. 2 STCF
Eucalyptus tereticornis NT
Eucalyptus tetrapleura NT
Eucalyptus tindaliae NT
Eucalyptus torquata NT
Eucalyptus umbra NT
Euchiton sphaericus NT
Eugenia reinwardtiana NT
Eupomatia bennetti NT
Eupomatia laurina NT.
Euroschinus falcatus NT
Eurychorda complanata
Eustrephus latifolius STVC
Everistia vacciniifolia var. nervosa NT
Evolvulus alsinoides NT
Excoecaria agallocha NT- BM
Exocarpos cupressiformis NT
Exocarpos latifolius NT
Exocarpos strictusNT
Exocarya sclerioides NTFagraea cambagei NTFaradaya splendida NTFicinia nodosa NTFicus benjamina NTFicus copiosaFicus crassipesFicus oppositaFicus coronata NTFicus fraseri NNTFicus hispida NTFicus macrophylla NTFicus microphylla var hilli NTFicus obliqua NTFicus racemosa NTFicus rubiginosa NTFicus sp. 1 NTFicus virens NTFicus watkinsiana NTFieldia australis NTFimbristylis ovata NTFimbristylis cinnamometorum NTFimbristylis dichotoma NTFimbristylis ferruginea NTFimbristylis nutans NTFimbristylis polytrichoides NTFimbristylis tristachya NTFimbristylis velata NTFlagellaria indica NTFlindersia australis NTFlindersia bennettiana NTFlindersia collina NTFlindersia schottiana NTFloydia praealta NTFreycinettia excelsa NT
Freycinettia marginata NT
Fuirena ciliaris NT
Gahnia aspera SA
Gahnia clarkei SA
Gahnia melanocarpa SA
Gahnia sieberiana SA
Gahnia subaequiglumis SA
Galium leptogonium NT
Gallionella ferruginea
Garcinia warrenii NT
Gaultheria appressa STF
Gaultheria viridicarpa STF
Geijera salicifolia NT
Geissois benthamiana NT
Geitonoplesium cymosum STVC
Genoplesium fimbriatum NT
Genoplesium rufum NT
Geodorum densiflorum ORCHID
Geranium homeanum NT
Geranium neglectum NT
Geranium solanderi NT
Gleichenia mendellii FERN
Gleichenia microphylla FERN
Glinus oppositifolius SA
Glochidion benthamianum SA
Glochidion ferdinandi subsp. ferdinandi SA
Glochidion ferdinandi subsp. pubens SA
Glochidion sumatranum SA
Glossocardia bidens STVC
Glossodia major ORCHID
Glossodia minor ORCHID
Glycine clandestina SA
Glycine cyrtoloba SA
Glycine microphylla SA
Glycine tabacina SA
Glycine tomentella SA
Glycyrrhiza acanthocarpa SA
Gmelina dalrympleana NT
Gmelina fasciculiflora NT
Gmelina leichhardtii NT
Gompholobium latifolium SA
Gompholobium pinnatum SA
Gompholobium virgatum SA
Gonocarpus chinensis NT
Gonocarpus micranthus NT
Gonocarpus oreophilus NTN
Goodenia bellidifolia subsp. argentea NT
Goodenia bellidifolia subsp. bellidifolia NT
Goodenia glabra NT
Goodenia grandiflora NT
Goodenia hederacea sub sp. hederacea STVC
Goodenia heterophylla sub sp eglandulosa, sub sp. heterophylla NT
Goodenia ovata NT
Goodenia paniculata NT
Goodenia rotundifolia NT
Goodenia stelligera NT
Goodenia sp.1 NT
Goodia lotifolia NT
Gossia acmenoides NT
Gossypium sturtianum STVC
Grammitis billardierei FERN
Grammitis stenophylla FERN
Graptophyllum excelsum NTN
Graptophyllum ilicifolium NTN
Gratiola pedunculata NT
Grevillea acanthifolia NT
Grevillea acuaria NT
Grevillea agrifolia NT
Grevillea albiflora NT
Grevillea alpina STF
Grevillea angulata NT
Grevillea arenaria STVC
Grevillea armigera NT
Grevillea asparagoides NT
Grevillea asplenifolia STVC
Grevillea asteriscosa STVC
Grevillea aurea STVC
Grevillea banyabbaNT
Grevillea baileyana STVC
Grevillea banksii NT
Grevillea baurii NT
Grevillea beadleana STVC
Grevillea bedggoodiana STVC
Grevillea bipinnatifida STVC
Grevillea biternata SA
Grevillea bronwenae STVC
Grevillea buxifolia STVC
Grevillea caleyi STVC
Grevillea calliantha NT
Grevillea candelabroides NT
Grevillea capitellata NT
Grevillea centristigma NT
Grevillea crithmifolia NT
Grevillea chrysophaea NT
Grevillea diffusa NT
Grevillea dimorpha NT
Grevillea disjuncta NT
Grevillea dissecta NT
Grevillea drummondii NT
Grevillea dryophylla NT
Grevillea eriobotrya NT
Grevillea eryngioides NT
Grevillea evansiana SA
Grevillea exposita NT
Grevillea fasciculata NT
Grevillea flexuosa NT
Grevillea floribunda STVC
Grevillea formosa NT
Grevillea fulgens NT
Grevillea georgeana STVC
Grevillea glauca STVC
Grevillea goodie NT
Grevillea granulifera NT
Grevillea heliosperma NT
Grevillea helmsiae NT
Grevillea hilliana NT
Grevillea hirtella STVC
Grevillea hookeriana SA
Grevillea humifusa NT
Grevillea humilis subsp. humilis NT
Grevillea humilis subsp.lucens NT
Grevillea humilis subsp. maritina NT
Grevillea iaspicula NT
Grevillea ilicifolia STVC
Grevillea incrassata NT
Grevillea infundibularis NT
Grevillea insignis NT
Grevillea involucrate NT
Grevillea irrasa NTN
Grevillea jephcottii STVC
Grevillea johnsonii STVC
Grevillea juncifolia NT
Grevillea juniperina STVC
Grevillea kennedyana SA
Grevillea lanigera STVC
Grevillea laurifolia STVC
Grevillea lavandulacea STVC
Grevillea leptobotrys NT
Grevillea linearifolia NT
Grevillea linsmithii NT
Grevillea longifolia NT
Grevillea longistyla H.
Grevillea macleayana NT
Grevillea masonii NT
Grevillea microstegia NT
Grevillea miniata NT
Grevillea mollis NT
Grevillea molyneuxi STVC
Grevillea mucronulata NT
Grevillea nana STVC
Grevillea nematophylla NT
Grevillea neurophylla NT
Grevillea nudiflora NT
Grevillea oleoides NT
Grevillea olivacea NT
Grevillea oncogyne NT
Grevillea paradoxa NT
Grevillea parallela NT
Grevillea patulifolia NT
Grevillea petrophiloides NT
Grevillea pluricaulis NT
Grevillea polybractea NT
Grevillea prasina STVC
Grevillea preissi STVC
Grevillea pteridifolia NT
Grevillea quadricauda NT
Grevillea quercifolia NT
Grevillea ramosissima NT
Grevillea renwickiana NT
Grevillea repens NT
Grevillea rhizomatosa STVC
Grevillea rhyolitica STVC
Grevillea rigida NT
Grevillea robusta NT
Grevillea rosmarinifolia STVC
Grevillea scortechinii STVC
Grevillea secunda STVC
Grevillea sericea NT
Grevillea sessilis NT
Grevillea shiressii NT
Grevillea speciosa NT
Grevillea sp.1 NT
Grevillea sp.2 NT
Grevillea stenobotrya SA
Grevillea stenomera NT
Grevillea striata NT
Grevillea tenuiflora NT
Grevillea thelemanniana NT
Grevillea thyrsoides NT
Grevillea treueriana NT
Grevillea trifida NT
Grevillea triloba NT
Grevillea tripartita subsp. macrostylis NT
Grevillea venusta NT
Grevillea victoriae NT
Grevillea whiteana NT
Grevillea wickhamii NT
Grevillea wilkinsonii NT
Grevillea wilsonii NT
Grewia latifolia NT
Guioa semiglauca NT
Guilfoylia monostylis NT
Gymnoschoenus sphaerocephalus NT
Gymnostachys anceps NT
Gymnostoma australianum NT
Haemodorum austroqueenslandicum NT-BM
Haemodorum planifolium NT-BM
Hakea actites NT
Hakea bakeriana NT.
Hakea bucculenta NT
Hakea clavata NT
Hakea eriantha NT
Hakea florulenta NT
Hakea laevipes subsp. laevipes NT
Hakea laurina NT
Hakea ochroptera NT
Hakea orthorrhyncha NT
Hakea pedunculata NT
Hakea purpurea NT
Hakea salicifolia NT-BM
Hakea sericea NT
Hakea subsulcata NT
Hakea teretifolia sub sp. teretifolia NT
Hakea verrucosa NT
Hakea victoria NT
Halfordia kendack NT
Halophila ovalis BM
Haloragis exalata var. velutina NT
Hardenbergia comptoniana HW
Hardenbergia violacea H,T,S.
Harnieria hygrophiloides NT
Harpullia arborea NT
Harpullia hillii NT
Harpullia pendula NT
Hedycarya angustifolia NT
Helichrysum rutidolepis NT,STVC
Helicia ferruginea NT
Helicia glabriflora NT
Helmholtzia glaberrima NT
Hemarthria uncinata subsp. spathata NT
Hemarthria uncinata subsp. uncinata NT
Heritiera trifoliolata NT
Heteropogan contortus NT
Hibbertia acicularis NT
Hibbertia acuminata NT
Hibbertia aspera NT
Hibbertia banksii NTN
Hibbertia dentata NT
Hibbertia diffusa NT
Hibbertia fasciculata NT
Hibbertia hermanniifolia NT
Hibbertia linearis NT
Hibbertia obtusifolia NT
Hibbertia pedunculata NT
Hibbertia riparia NTT
Hibbertia rufa NT, STVC, BM
Hibbertia salicifolia NT- BM
Hibbertia scandens NT
Hibbertia serpyllifolia NT
Hibbertia vestita NT
Hibbertia villosa NT
Hibiscus diversifolia NT
Hibiscus geranioides NT
Hibiscus heterophyllus NT-BM
Hibiscus insularis NT
Hibiscus macilwraithensis NT
Hibiscus sp. barambah creek NT
Hibiscus splendens NT
Hibiscus sturtii NT
Hibiscus trionum NT
Hibiscus tozerensis NT
Hicksbeachia pinnatifolia SA
Hippocratea barbata NT
Histiopteris incisa FERN
Hodgkinsonia ovatiflora NT
Homoranthus flavesence NT
Homoranthus virgatus NT
Homalanthus novoguineensis NT
Homalanthus populifolius NT
Hovea acutifolia NT
Hovea graniticola STVC
Hovea heterophylla STVC
Hovea ramulosa STVC
Hovea similis NT
Howea forsteriana NT
Howittia trilocularis NT
Hoya australis NT
Hoya macgillivrayi NT
Hoya oligotricha NT
Huperzia carinata AS PER FERNS
Huperzia phlegmaria AS PER FERNS
Huperzia phegmarioides AS PER FERNS
Hybanthus monopetalus NT
Hybanthus stellarioides NT
Hybanthus vernonii subsp. scaber NT
Hybanthus vernonii subsp. veronii NT
Hydriastele wendlandiana NT
Hydrilla verticillata SUB
Hydrocharis dubia BM
Hydrocotyle acutiloba BM
Hydrocotyle geraniifolia BMBM
Hydrocotyle pedicellosa BM
Hydrocotyle peduncularis BM
Hydrocotyle tripartita BM
Hydrocotyle verticillata BM
Hymenophyllum australe FERN
Hymenophyllum bivalve FERN
Hymenophyllum cupressiforme FERN
Hymenophyllum flabellatum FERN
Hymenosporum flavum NT
Hyparrhenia filipendula NT
Hypericum gramineum NT
Hypoestes floribunda BM
Hypolaena fastigiata BM
Hypolepis glandulifera BM
Hypolepis muelleri BM
Hypoxis pratensis BM
Imperata cylindrica NT
Indigofera australis TW
Indigofera hirsuta TW
Indigofera linifolia TW
Indigofera pratensis TW
Intsia bijuga NT
Iphigenia indicaNT
Ipomoea brasiliensis SA
Ipomoea plebeian SA
Isachne globosaNT
Ischaemum australe subsp. australe,NT
Ischaemum australe subsp. villosum NT
Ischaemum triticeum NT
Isolepis cernua BM
Isolepis habra BM
Isolepis inundata BM
Isolepis subtilissima STVC, BM
Isopogon anemoniifolius NT
Isopogon cuneatus NT
Isopogon dubius NT
Isopogon mnoraifolius NT
Isopogon petiolaris NT
Isotoma armstrongii NT
Isotoma axillaris STVC
Isotoma fluviatilis subsp.borealis STVC
Isotoma fluviatilis subsp. fluviatilis STVC
Ixora bleckeri NT
Jacksonia chappilliae HW
Jacksonia scoparia HW
Jacksonia stackhousei HW
Jagera pseudorhus subsp. pseudorhus NT
Jasminum didymum NT
Jasminum singuliflorum NT
Jasminum simplicifolium subsp. suavissimum NT
Jasminum volubile NT
Juncus bufonius BM
Juncus continuus BM
Juncus kraussii BM
Juncus mollis BM
Juncus planifolius BM
Juncus polyanthemus BM
Juncus prismatocarpus BM
Juncus saraphorus BM
Juncus subsecundus BM
Juncus usitatus BM
Juncus vaginatus BM
Kardomia prominens NT
Kardomia silvestris NT
Kennedia nigricans HW
Kennedia rubicunda HW
Kennedia ??????? HW
Keraudrenia corollata subsp. corollata NT
Keraudrenia corollata subsp. denticulata NT
Keraudrenia hillii sub sp. hillii NT
Kopsia arborea NT
Korthalsella rubra sub sp. geijericola STVC
Korthalsella rubra subsp. rubra GSTVC
Kunzea affinis NT
Kunzea ambigua NT
Kunzea bracteolata NT, STVC
Kunzea capitata NT
Kunzea opposita NT
Kunzea parvifolia NT, STVC
Kunzea pauciflora NT
Lachnagrostis billardierei NT
Lachnagrostis filiformis NT
Laccospadix australasicus NT
Lagunaria pattersonii NT, SA
Lambertia echinata NT
Lambertia formosa NT
Landoltia punctata
Larsenaikia ochreata NT
Lasiopetalum ferrugineum NT
Lastreopsis acuminate FERN
Lastreopsis decomposita FERN
Lastreopsis marginans FERN
Lastreopsis microsora FERN
Lastreopsis munita FERN
Laxmannia gracilis NT
Lechenaultia biloba NT
Lechenaultia formosum NT
Leea indica NT
Leersia hexandra NT
Legnephora moorei NT
Leiocarpa serpens NT, STVC
Leionema elatius subsp. beckleri NT
Leionema elatius subsp. elatius NT
Lemna trisulca NT
Lepiderema pulchella NT
Lepidosperma concavum NT
Lepidosperma elatius NT
Lepidosperma filiforme NT
Lepidosperma gunnii NT
Lepidosperma latens NT
Lepidosperma laterale NT
Lepidosperma limicola STVC
Lepidosperma longitudinale NT
Lepidosperma neesii NT
Lepidosperma quadrangulatum NT
Lepidosperma tortuosum STVC
Lepidosperma urophorum NT
Lepidozamia hopei SA
Lepidozamia peroffskyana SA
Lepironia articulata NT
Leptocarpus tenax NT
Leptomeria acida NT
Leptomeria drupacea NT
Leptospermum arachnoides NT
Leptospermum benwellii NT
Leptospermum brachyandra NT
Leptospermum brevipes NT
Leptospermum gregarium NT
Leptospermum juniperinum NT
Leptospermum laevigatum NT
Leptospermum leuhmannii NT
Leptospermum liversidgei NT
Leptospermum madidum subsp. madidum NT
Leptospermum madidum subsp. sativum NT
Leptospermum microcarpum NT
Leptospermum minutifolium STVC
Leptospermum novae-angliae STVC
Leptospermum petersonii NT
Leptospermum polygalifolium NT
Leptospermum rotundifolium NT
Leptospermum scoparium NT
Leptospermum semibaccatum NT
Leptospermum speciosum NT
Leptospermum squarrosum NT
Leptospermum trinervium NT
Leptospermum variabile NT
Leptospermum whitei NT
Lepyrodia imitans NT
Lepyrodia scariosa NT
Lespedeza juncea subsp. sericea NT
Leucochrysum albicans subsp. albicans NT
Leucopogon biflorus NT
Leucopogon deformis NT
Leucopogon ericoides NT
Leucopogon esquamatus NT
Leucopogon juniperinus NT
Leucopogon lanceolatus NT
Leucopogon leptospermoides NT
Leucopogon margarodes NT
Leucopogon melaleucoides STVC
Leucopogon neoanglicus STVC
Leucopogon parviflorus NT
Leucopogon recurvisepalus NT
Leucopogon rodwayi NT
Leucopogon trichostylus STVC
Leucopogon virgatus NT
Libertia paniculata NT
Licuala ramsayi NT
Ligustrum australianum NT
Lindsaea dimorpha FERN
Lindsaea incisa FERN
Lindsaea linearis FERN
Lindsaea microphylla FERN
Linospadix minor NT, SA
Linospadix monostachya NT, SA
Liparophyllum exaltatum NT
Lipocarpha microcephala NT
Litsea australis NT
Litsea reticulata NT
Livistona australis SA
Livistona benthamii SA
Livistona concinna SA
Livistona decora SA
Livistona drudei SA
Livistona humilis SA
Livistona inermis SA
Livinstona lanuginosa SA
Livistona mariae SA
Livistona muelleri SA
Livistona rigida SA
Livistona victoriae SA
Lobelia anceps NT
Lobelia andrewsii NT
Lobelia membranacea NT
Lobelia trigonocaulis NT
Logania albiflora NT
Logania pusilla NT
Lomandra banksii SA
Lomandra confertifolia subsp. pallida SA, STVC
Lomandra confertifolia subsp. rubignosa SA, STVC
Lomandra elongata SA, STVC
Lomandra filiformis subsp. coriacea SA, STVC
Lomandra filiformis subsp. filiformis SA, STVC
Lomandra filiformis subsp. flavior SA, STVC
Lomandra hystrix Scan leaves SA, STVC
Lomandra laxa SA, STVC
Lomandra leucocephala SA, STVC
Lomandra longifolia SA, STVC
Lomandra obliqua SA, STVC
Lomandra spicata SA, STVC
Lomatia arborescens NT
Lomatia fraseri STVC
Lomatia silaifolia STVC
Landoltia punctata
Lophostemon confertus NT
Lophostemon suaveolens NTN
Ludwigia octovalvis NT
Ludwigia peploides subsp. montividensis NT
Luffa aegyptiaca var. leiocarpa NT
Lunasia amara NT
Lycopodiella cernua AS PER FERNS
Lycopodiella lateralis AS PER FERNS
Lycopodiella serpentina AS PER FERNS
Lycopodium deuterodensum AS PER FERNS
Lycopus australis NT
Lygodium japonicum FERN
Lygodium microphyllum FERN
Lyperanthus suaveolens ORCHID
Lysimachia japonica NT
Lythrum hyssopifolia BM
Lythrum salicaria BM
Macadamia integrifolia SSA
Macadamia tetraphylla SA
Macaranga involucrate NT
Macaranga tanarius NT
Macarthuria neocambrica NT
Mackinlaya macrosciadea NT
Maclura cochinchinensis NT
Macrothelypteris torresiana FERN
Macrozamia cairnsiana SA
Macrozamia communis SA
Macrozamia fawcettii SA
Macrozamia johnsonii SA
Macrozamia lucida SA
Macrozamia macdonnellii SA
Macrozamia miquelii SA
Macrozamia mooreii SA
Macrozamia pauli-guilielmi SA
Maireana brevifolia SA
Mallotus claoxyloides var. ficifolius NT
Mallotus discolor NT
Mallotus philippensis NT
Mallotus polyadenos NT
Malvastrum coromandelianum NT
Manilkara kauki NT
Maniltoa lenticellata NT
Marsdenia flavescens NT
Marsdenia fraseri NT
Marsdenia hemiptera NT
Marsdenia liisae NT
Marsdenia lloydii NT
Marsdenia longiloba NT
Marsdenia rostrata NT
Marsilea drummondii FERN
Marsilea hirsuta FERN
Marsilea mutica FERN
Maundia triglochinoides NT
Maytenus bilocularis SA
Maytenus silvestris NT
Mazus pumilio DIV, NT
Megahertzia amplexicaulis NT
Meiogyne stenopetala NT
Melaleuca acuminata NT
Melaleuca acuminatus NT
Melaleuca alsophila NT
Melaeuca alternifolia NT
Melaleuca argentea NT
Melaleuca armillaris NT
Melaleuca bracteata NT, BM
Melaleuca citina NT
Melaleuca Comboyensis NT
Melaleuca decora NT
Melaleuca densa NT
Melaleuca formosus NT.
Melaleuca hypericifolia NT
Melaleuca irbyana NT, BM
Melaleuca lateritia NT
Melaleuca leucadendra NT, BM
Melaleuca linariifolia NT,BM
Melaleuca microphylla NT
Melaleuca nesophila NT
Melaleuca nodosa NT, BM
Melaleuca pachyphyllus NT,BM
Melaleuca pallidus STVC
Melaleuca pearsonii NT
Melaleuca polandii NT
Melaleuca quinquinervia NT, BM
Melaleuca rigidus NT
Melaleuca salicina NT, BM
Melaleuca sieberiNT
Melaleuca spathulata NT
Melaleuca sp1 NT
Melaleuca sp. hinchinbrook NT
Melaleuca squamea NT.
Melaleuca squarrosa NT
Melaleuca stypheloides NT, BM
Melaleuca subulatus NT
Melaleuca tamariscina NT, BM
Melaleuca thymifolia NT, BM
Melaleuca uncinata NT
Melaleuca viminalis NT, BM
Melaleuca viridiflora NT, BM
Melaleuca williamsii STVC
Melanthera biflora NT
Melastoma affine NT, BM
Melia azedarach H, L, S, G.
Melichrus adpressus NT
Melichrus hirsutus NT
Melichrus procumbens NT
Melichrus sp. Gibberagee NT
Melichrus urceolatus NT
Melicope elleryana NT
Melicope hayesii NT
Melicope micrococca NT
Melicytus dentatus STVF
Melodinus acutiflorius/Melodinus guilfoylei NT
Melodinus australis NT
Melodinus guilfoylei/Melodinus acutiflorus NT
Mentha diemenica DIV, NT
Mentha satureioides DIV NT
Micrantheum ericoides NT
Micrantheum hexandrum STVC
Microcitrus australasica NT
Microlaena stipoides NT
Micromelum minutum NT
Microsorum pustulatum FERN
Microsorum scandens FERN
Microtis parviflora ORCHID
Mirbelia rubiifolia NT
Mischocarpus anodontus NT
Mischocarpus australis NT
Mischocarpus pyriformis NT
Mitrasacme alsinoides NT
Mitrasacme polymorpha NT
Momordica balsamina STVC
Momordica charantia STVC
Monococcus echinophorus NT
Monotoca scoparia NT
Morinda canthoides NT
Morinda citrifolia NT
Morinda jasminoides NT
Mucuna gigantean sub sp. gigantea SA
Muehlenbeckia auxillaris STVF
Muelenbeckia gracillima STVC
Muellerina celastroides NT
Muellerina eucalyptoides NT
Murdannia graminea SA
Musa banksii SA
Myoporum acuminatum NT
Myoporum betcheanum NT
Myoporum boninense var. australe NT
Myoporum montanum NT
Myriophyllum crispatum BM
Myriophyllum gracile subsp. gracile BM
Myriophyllum gracile subsp. lineare BM
Myriophyllum latifolium BM
Myriophyllum variifolium BM, SUB
Myriophyllum verrucosum BM
Myristica insipida NT
Myrmecodia beccarii NT
Myrsine benthamiana NT
Myrsine howittiana NT
Myrsine variabilis NT
Najas marina BM
Nauclea orientalis. NT
Neisosperma poweri NT
Nelumbo nucifera BM
Nematolepis squamea NT
Neoachmandra cunninghamii NT
Neofabricia myrtifolia NT
Neolitsea australiensis NT
Neolitsea dealbata NT
Neorites kevedianus NT
Nepenthes mirabilis BM
Nepenthes rowanae BM
Nephrolepis arida FERN
Nephrolepis biserrata FERN
Nephrolepis cordifolia FERN
Nephrolepis hirsutula FERN
Nicotiana forsteri NT
Niemeyera whitei NT
Normanbya normanbyi SA
Notelaea johnsonii NT
Notelaea longifolia subsp. glabra NT
Notelaea longifolia subsp. intermedia NT
Notelaea longifolia subsp. longifolia NT
Notelaea ovata NT
Notelaea venosa NT
Nothofagus moorei NT
Notothixos cornifolius NT
Notothixos incanus NT
Notothixos subaureus NT
Nymphaea gigantea SUB
Nymphaea violacea SUB
Nymphoides geminata SUB
Nymphoides indica SUB
Nyssanthes diffusa NT
Oberonia complanata ORCHID
Oberonia titania ORCHID
Ochrosperma citriodorum NT
Ochrosperma lineare NT
Olax angulata NT
Olax retusa NT
Olax stricta NT
Olea paniculata SA
Olearia argophylla NT
Olearia canescens STVC
Olearia cydoniifolia STVC
Olearia elliptica STVC
Olearia erubescens NT
Olearia flocktoniae STVC
Olearie nernstii STVC
Olearia stilwelliae NT
Opercularia hispida NT
Ophioglossum pendulum FERN
Ophioglossum reticulatum FERN
Oplismenus aemulus NT
Oplismenus imbecillis NT
Oplismenus undulatifolius NT
Orites excelsus NT
Ormosia ormondii NT
Orthoceras strictum NT
Orthosiphon aristatus NT
Osbeckia australiana NT
Ottelia ovalifolia subsp. ovalifolia NT
Ottelia ovalifolia subsp. chrysobasis NT
Ottochloa gracillima NT
Owenia cepiodora NT
Oxalis chnoodes NT
Oxalis exilis NT
Oxalis perennans NT
Oxalis radicosa NT
Oxalis rubens NT
Oxylobium arborescens HW
Oxylobium robustum HW
Ozothamnus bidwillii NT
Ozothamnus diosmifolius NT
Ozothamnus obcordatus NT
Ozothamnus obovatus NT
Ozothamnus rufescens NT
Ozothamnus thyrsoideusNT
Ozothamnus whitei NT
Palmeria scandens NT
Pandanus conicus SA
Pandanus lauterbachii SA
Pandanus monticola SA
Pandanus solmslaubachii SA
Pandanus tectorius SA
Pandorea jasminoides NT
Pandorea nervosa NT
Pandorea pandorana NT
Panicum bisulcatum NT
Panicum decompositum NT
Panicum effusum NT
Panicum lachnophyllum NT
Panicum obseptum NT
Panicum paludosum NT, BM
Panicum pygmaeum NT
Panicum queenslandicum NT
Panicum simili NT
Papillilabium beckleri ORCHID
Paracaleana minor ORCHID
Pararchidendron pruinosum NT
Paristolochia praenevosa NT
Parsonsia dorrigoensis NT
Parsonsia fulva NT
Parsonsia induplicata NT
Parsonsia lanceolata NT
Parsonsia longipetiolata NT
Parsonia sp.1 NT
Parsonsia straminea NT
Parsonsia velutina NT
Paspalidium aversum NT
Paspalidium criniforme NT
Paspalidium distans NT
Paspalidium gausum NT
Paspalidium grandispiculatum NT
Paspalum distichum NT
Paspalum orbiculare NT
Paspalum scrobiculatum NT
Paspalum vaginatum NT
Passiflora aurantia NT
Passiflora cinnabarina NT
Passiflora herbertiana NT
Patersonia glabrata NT
Patersonia longifolia NT
Patersonia sericea NT
Pavetta australiensis NT
Pelargonium australe NT
Pelargonium helmsii NT
Pelargonium inodorum NT
Pelargonium rodneyanum NT
Pellaea falcata FERN.
Pellaea nana FERN
Pellaea paradoxa FERN
Peltophorum pterocarpum HW
Pennantia cunninghamii NT
Pennisetum alopecuroides NT
Pentaceras australe NT
Peperomia blanda var. floribunda NT
Peperomia tetraplylla NT
Peristeranthus hillii ORCHID
Persicaria attenuata NT, BM
Persicaria decipiens NT, BM
Persicaria dichotoma NT, BM
Persicaria elatior NT, BM
Persicaria hydropiper NT, BM
Persicaria lapathifolia NT, BM
Persicaria orientalis NT, BM
Persicaria praetermissa NT, BM
Persicaria strigosa NT, BM
Persicaria subsessilis NT, BM
Persoonia katerae SEE SPECIES PROPAGATION.
Persoonia media SEE SPECIES PROPAGATION.
Persoonia oleoides SEE SPECIES PROPAGATION.
Persoonia sericea SEE SPECIES PROPAGATION.
Persoona stradbrokensis SEE SPECIES PROPAGATION.
Persoona tenuifolia SEE SPECIES PROPAGATION.
Persoonia virgata SEE SPECIES PROPAGATION.
Petalostigma pubescens SA
Petalostigma triloculare SA
Petermannia cirrosa NT
Petrophile canescens NT
Petrophile pulchella NT
Phaius australis ORCHID
Phebalium nottii NT
Phebalium squamulosum subsp. argenteum NT
Phebalium squamulosum subsp. squamulosum NT
Phebalium squamulosum subsp.verrucosum NT
Phebalium squamulosum subsp. argenteum NT
Phebalium squamulosum subsp. squamulosum NT
Phebalium squamulosum subsp. verrucosum NT
Phebalium woombye NT
Philotheca conduplicata NT
Philotheca difformis subsp. smithiana STVC
Philotheca myoporoides STVC
Philotheca papillata STVC
Philotheca salsolifolia subsp. pedicellata NT
Philotheca salsolifolia subsp.salsolifolia NT
Philotheca verrucosus NT
Philydrum lanuginosum BM
Phragmites australis BM
Phyllachne colensoi DIV, STF
Phyllanthus cuscutiflorus STVC
Phyllanthus gunnii NT
Phyllanthus hirtellus NT
Phyllanthus microcladus NT
Phyllanthus similis NT
Phyllanthus subcrenulatus NT
Phyllota phylicoides NT
Pilidiostigma glabrum NT
Pimelea glauca NT
Pimelea latifolia subsp. altior NT
Pimelea latifolia subsp. elliptifolia NT
Pimelea latifolia subsp. hirsute NT
Pimelea latifolia subsp. latifolia NT
Pimelea ligustrina subsp. ciliate NT
Pimelea ligustrina subsp. hypericina NT
Pimelea ligustrina subsp. ligustrina NT
Pimelea linifolia subsp. caesia NT
Pimelea linifolia subsp. linifolia NT
Pimelea linifolia subsp. linoides NT
Pimelea neo-anglica NT
Piper hederaceum subsp. hederaceum NT
Piper hederaceum subsp. longiorispicum NT
Pipturus argenteus NT
Pisonia aculeata NT
Pisonia grandis NT
Pisonia umbellifera NT
Pittosporum angustifolium NT
Pittosporum lancifolium NT
Pittosporum multiflorum NT
Pittosporum phylliraeoides NT
Pittosporum revolutum NT
Pittosporum undulatum NT
Pittosporum venulosum NT
Planchonella australis SA
Planchonella chartacea SA
Planchonella pohlmaniana SA
Plantago debilis NT
Platycerium bifurcatum FERN
Platycerium superbum FERN
Platycerium veitchii FERN
Platylobium formosum HW
Platysace ericoides NT
Platysace heterophylla NT
Platysace lanceolata NT
Platysace stephensonii NT
Plectorrhiza tridentata ORCHID
Plectranthus alloplectus NT
Plectranthus apreptus NT
Plectranthus argentatus NT
Plectranthus bellus NT
Plectranthus cremnus NT
Plectranthus graniticola NT
Plectranthus gratus NT
Plectranthus graveolens NT
Plectranthus nitidus NT
Plectranthus parviflorus NT
Plectranthus sauveolens NT
Pleomele angustifolia NT
Plumbago zeylanica NT
Pneumatopteris sogerensis FERN
Poa billardierei NT
Poa labillardierei subsp. acris NT
Poa labillardierei subsp. labillardier NT
Poa queenslandica NT
Poa sieberiana subsp. hirtella & subsp. sieberiana NT
Poa sieberiana subsp. sieberiana NT
Poa sp.1 NT
Podocarpus elatus SA
Podocarpus lawrencei SA
Podolepis neglecta NT
Podolobium aciculiferum HW, SA
Podolobium aestivum HW, SA
Podolobium ilicifolium HW, SA
Podolobium scandens HW, SA
Pollia crispata NT
Polyalthia nitidissima NT, SA
Polycarpaea corymbosa NT
Polygala japonica TW
Polygala linariifolia TW
Polymeria calycina TW
Polyosma cunninghamii NT
Polyscias elegans NT
Polyscias murrayi NT
Polyscias sambucifolia subsp. decomposita NT
Polyscias sambucifolia subsp. sambuafolia NT
Polystichum australiensis FERN
Polystichum formosum FERN
Polystichum proliferum FERN
Pomaderris argyrophylla NT
Pomaderris eriocephala NT
Pomaderris ferruginea NT
Pomaderris intermedia NT
Pomaderris lanigera NT
Pomaderris ledifolia STVC
Pomaderris ligustrina subsp. ligustrina STVC
Pomaderris nitidula NT
Pomaderris prunifolia subsp. prunifolia NT
Pomaderris subcapitata NT
Pomax umbellata NT
Poranthera corymbosa NT
Poranthera ericifolia NT
Poranthera microphylla NT
Poranthera obovata NT
Portulaca oleracea NT
Potamogeton crispus NT
Potamogeton octandrus BM
Potamogeton perfoliatus BM
Potamogeton tricarinatus MB
Potamophila parviflora NT
Pothos longipes NT
Pouteria eerwah SA
Pouteria papyracea SA
Pouteria queenslandica SA
Prasophyllum australe ORCHID
Prasophyllum brevilabre ORCHID
Prasophyllum elatum ORCHID
Prasophyllum flavum ORCHID
Prasophyllum patens ORCHID
Pratia pedunculata DIV
Pratia purpurascencs DIV
Premna lignum-vitae SA
Priophys amboidensis NT
Priophys cunninghammii NT
Prostanthera caerulea NT
Prostanthera incisa NTN
Prostanthera lanceolata NT
Prostanthera lasianthos STF
Prostanthera nivea subsp. nivea NT
Prostanthera nivea subsp. induta NT
Prostanthera ovalifolia NT
Prostanthera palustris NT
Prostanthera prunelloides NT
Prostanthera rhombea NT
Prostanthera rotundifolia NT
Prostanthera scutellarioides NT
Prostanthera sejuncta NT
Prumnopitys laei NT
Pseudanthus orientalis SA
Pseudanthus pauciflorus SA
Pseuderanthemum variabile NT
Pseudognaphalium luteoalbum NT
Pseudoraphis paradoxa NT
Pseudovanilla foliata ORCHID with inoculation
Pseudoweinmannia lachnocarpa NT
Pseuduvaria froggattii SA
Pseuduvaria villosa SA
Psilotum complanatum FERN
Psilotum nudum FERN
Psychotria daphnoides NT
Psychotria loniceroides NT
Psychotria simmondsiana NT
Psydrax attenuata NT
Psydrax lamprophylla var. lamprophylla NT
Psydrax odorata subsp. australiana NT
Psydrax odorata subsp. buxifolia NT
Pteridium esculentum FERN
Pteris comons FERN
Pteris ensiformis FERN
Pteris tremula FERN
Pteris umbrosa FERN
Pteris vittata FERN
Pterocaulon redolens NT
Pterostylis baptistii ORCHID
Pterostylis curta ORCHID
Pterostylis daintreana ORCHID
Pterostylis erecta ORCHID
Pterostylis fischii ORCHID
Pterostylis furcata ORCHID
Pterostylis grandiflora ORCHID
Pterostylis hildae ORCHID
Pterostylis hispidula ORCHID
Pterostylis longifolia ORCHID
Pterostylis mutica ORCHID
Pterostylis nutans ORCHID
Pterostylis obtusa ORCHID
Pterostylis ophioglossa ORCHID
Pterostylis parviflora ORCHID
Pterostylis pedunculata ORCHID
Pterostylis russellii ORCHID
Ptilothrix deusta STVC
Ptilotus exaltus NT
Ptilotus macrocephalis NT
Ptilotus mangelsii NT
Ptilotus obovatus NT
Ptychospermum elegans SA
Ptychospermum macarthurii SA
Pultenaea alea HW, SA
Pultenaea daphnoides HW, SA
Pultenaea dentata HW, SA
Pultenaea euchila HW, SA
Pultenaea flexilis HW, SA
Pultenaea maritima HW, SA
Pultenaea myrtoides HW, SA
Pultenaea petiolaris STCV-HW, SA
Pultenaea pycnocephala STCV-HW, SA
Pultenaea retusa HW, SA
Pultenaea robusta HW, SA
Pultenaea rostrata HW, SA
Pultenaea spinosa HW, SA
Pultenea villosa HW, SA
Pyrossia confluens T,S,G. N.G.
Pyrossia lanceolata FERN
Pyrossia longifolia FERN
Pyrossia rupestris FERN
Quassia sp. mooney creek SA
Quassia sp. mount nardi SA
Quintinia sieberi SA
Quintinia verdonii SA
Ranunculus inundatus NT
Ranunculus lappaceus NT.
Ranunculus plebeius NT
Regelia megacephala NT
Regelia velutina NT
Remusatia vivipara
Rhagodia candolleana subsp. candolleana SA
Rhaphiodophora australasica NT
Rhaphiodophora sp.1 NT
Rhinerrhiza divitiflora ORCHID
Rhizophora stylosa ORCHID
Rhodamnia argenta NT
Rhodamnia rubescens NT
Rhodanthe anthemoides NT
Rhodanthe chlorocephala subsp. chlorocephala NT
Rhodanthe chlorocephala subsp. rosea NT
Rhododendron viriosum STVC
Rhodomyrtus psidioides NT
Rhodosphaera rhodanthema NT
Rhynchospora brownie NT
Rhysotoechia bifoliolata NT
Rhytidosporum procumbens NT
Ricinocarpos pinifolius SA
Ricinocarpus speciosus SA
Ripogonum album NT
Ripogonum brevifolium NT
Ripogonum discolor NT
Ripogonum elseyanum NT
Ripogonum fawcettianum NT
Rorippa dictyosperma NT
Rorippa gigantea NT
Rorippa laciniata NT
Rubus moluccanus NT
Rubus nebulosus NT
Rubus parvifolius NT
Rubus probus NT
Rubus queenslandica NT
Rubus rosifolius NT
Rulingia dasyphylla STVC
Rulingia salviifolia STVC
Rumex brownii STVC
Rumohra adiantiformis FERN
Ruppia maritima SUB, BM
Rutidosis heterogama NT
Rytidosperma longifolium NT
Rytidosperma tenuius NT
Sacciolepis indica NT
Salacia chinensis NT
Salvia plebeia DIV
Sambucus australisica NT
Samolus repens DIV
Samolus valerandi DIV
Sannantha angusta NT
Sannantha collina NT
Sannantha similis NT
Santalum obtusifolium NT
Sarcochilus aequalis ORCHID
Sarcochilus australis ORCHID
Sarcochilus ceciliae ORCHID
Sarcochilus falcatus ORCHID
Sarcochilus fitzgeraldii ORCHID
Sarcochilus hillii ORCHID
Sarcochilus parviflorus ORCHID
Sarcochilus spathulatus ORCHID
Sarcocornia quinqueflora subsp. quinqueflora NT
Sarcomelicope simplicifolia subsp. simlicifolia NT
Sarcopetalum harveyanum SA, NT
Sarcopteryx stipata NT
Sarcotoechia lanceolata NT
Sarcotoechia serrata NT
Scaevola aemula NT
Scaevola albida NT
Scaevola calandulencea NT
Scaevola calliptera NT
Scaevola hookeri NT
Scaevola ramosissima NT
Scaevola tomentosa NT
Schelhammera undulata NT
Scheflerra actinophyla NT
Schistotylus purpuratus ORCHID
Schizaea bifida FERN
Schizaea dichotoma FERN
Schizaea rupestris FERN
Schizomeria ovata NT
Schizachyrium fragile NT
Schoenoplectus mucronatus NT, BM
Schoenoplectus subulatus NT, BM
Schoenoplectus validus NT, BM
Schoenus apogon NT
Schoenus brevifolius NT
Schoenus ericetorum NT
Schoenus maschalinus NT, BM
Schoenus melanostachys NT
Schoenus nitens NT
Schoenus pachylepis NT
Schoenus paludosus NT
Schoenus scabripes NT
Schoenus turbinatus NT
Schoenus vaginatus NT
Scirpus polystachyus STVC, BM
Scleranthus biflorus DIV
Scleria mackaviensis NT
Scleria rugosa NT
Scleria tricuspidata NT
Scolopia braunii HW
Secamone ellipticaNT
Selaginella uliginosa DIV
Senecio amygdalifolius NT
Senecio bipinnatisectusNT
Senecio diaschidesNT
Senecio linearifolius NT
Senecio magnificus NT
Senecio minimusNT
Senecio pinnatifolius subsp. pinnatifolius NT
Senecio pinnatifolius subsp. serratus NT
Senecio prenanthoidesNT
Senecio tenuiflorus NT
Senecio vagus subsp. eglandulosus NT
Senecio vagus subsp. vagus NT
Senegalia albizioides NT
Senna acclinis BW
Senna artemisioides subsp. artemisiodes BW
Senna artemisioides subsp. zygophylla BW
Senna cardiosperma BW
Senna charlesiana BW
Senna clavigera BW
Senna ferraria BW
Senna notabalis BW
Senna odorata BW
Senna stricta BW
Senna symonii BW
Senna venusta BW
Seringia arborescensNT
Sesbania cannabina HW
Sesuvium portulacastrumNT
Setaria australiensis NT
Sicyos australisNT
Sida cordifoliaNT
Sigesbeckia orientalis subsp. orientalis NT
Siphonodon australis SA
Sloanea australis NT
Sloanea woollsii NT
Smilax australis NT
Smilax glyciphylla NT
Solanum americanum NT
Solanum aviculare NT
Solanum campanulatum NT
Solanum centrale NT
Solanum corifolium NT
Solanum densevestitum NT
Solanum ditrichum NT
Solanum ellipticum NT
Solanum erianthum NT
Solanum gabrielae NT
Solanum hapalum NT
Solanum inaequilaterum NT
Solanum laciniatum NT
Solanum nobile NT
Solanum nummularium NT
Solanum opacum NT
Solanum parvifolium NT
Solanum petrophilum NT
Solanum plicatile NT
Solanum prinophylum NT
Solanum semiarmatum NT
Solanum stelligerum NT
Solanum symonii NT
Solanum vescum NT
Solanum vicinum NTN
Solenogyne bellioides NT
Sophora fraseri HW
Sophora tomentosa subsp.australis HW
Sophora tomentosa subsp. tomentosa HW
Sorghum leiocladum NT
Sowerbaea juncea NT
Sparganium subglobosum BM
Spartothamnella juncea NT
Spermacoce brachystema BM
Sphaerolobium vimineum HW
Sphagnum cristatum DIV
Spinifex sericeus NT
Spiranthes australis ORCHID
Spirodela polyrhiza
Sporadanthus caudatus NT
Sporadanthus interruptus NT
Sprengelia sprengelioidesNT
Sporobolus laxus H,T,L,F,S,G. NT
Sporobolus virginicus subsp. minor NT
Sporobolus virginicus subsp. virginicus NT
Spyridium scortechinii NT
Stackhousia spathulata NT
Stackhousia viminea NT
Stellaria angustifolia subsp. angustifolia NT
Stellaria angustifolia, subsp. rotundisepala NT
Stellaria angustifolia subsp. tenella NT
Stellaria flaccida NT
Stenocarpus salignus NT
Stenocarpus sinuatis NT
Stephania japonica var. discolor NT
Sterculia quadrifida NT
Sticherus flabellatus FERN
Sticherus lobatus FERN
Sticherus urceolatus FERN
Streblus pendulinus NT
Streptothamnus moorei NT
Strychnos arborea NT
Stuckenia pectinatus SUB
Stylidium debile NT
Stylidium graminifolium NT
Stylidium laricifolium NTN
Stylidium paniculatum TNT
Styphelia triflora HW
Styphelia viridis subsp. breviflora HW
Styphelia viridis subsp. viridis HW
Suaeda australis NT, DIV
Swainsona brachycarpa HW
Swainsona canescens HW
Swainsona galegifolia HW
Swainsona greyana HW
Swainsona maccullochiana HW
Swainsona procumbens HW
Swainsona queenslandica HW
Symplocos stawellii NNT
Symplocos thwaitesii NT
Syncarpia glomulifera subsp. glabra NT
Syncarpia glomulifera subsp. glomulifera NT
Synonum glandulosum NT
Syzygium alliiligneum NT
Syzygium aqueum NT
Syzygium australe NT
Syzygium buettnerianum NT
Syzygium corianthum NT
Syzygium cormiflorum NT
Syzygium crebrinerve NT
Syzygium cryptophlebium NT
Syzygium erythrocalyx NT
Syzygium erythrodoxum NT
Syzygium fibrosum NT
Syzygium forte subsp. potamophilum NT
Syzygium francisii NT
Syzygium hodgkinsoniae NT
Syzygium leuhmannii NT
Syzygium malaccense NT
Syzygium moorei NT
Syzygium oleosum NT
Syzygium paniculatum NT
Syzygium tierneyanum NT
Syzygium wilsonii NT
Tabernaemontana pandacaqu NT
Taeniophyllum muelleri NT
Talipariti tiliaceusis NT
Tapeinochilos ananassae DIV, NT
Tasmannia insipida STVC
Tasmannia stipitata STVC
Tecomanthe hillii NT, SA
Telopea aspera NT
Telopea speciossima NT
Tephrosia brachyodon HW
Tephrosia filipes HW
Tephrosia gordum-solice HW
Terminalia arenicola NT
Terminalia arostrata NT
Terminalia catappa NT
Terminalia muelleri NT
Terminala platyphylla NT
Ternstroemia cherryi SA
Tetracera nordtiana NT
Tetragonia tetragonioides SA
Tetrarrhena juncea BM
Tetrastigma nitens
Tetratheca juncea NT
Tetratheca thymifolia NT
Teucrium argutum NT
Teucrium corymbosum NT
Thaleropia queenslandica NT
Thelychiton falcorostrus ORCHID
Thelychiton kingianus ORCHID
Thelychiton speciosus ORCHID
Thelymitra angustifolia ORCHID
Thelymitra fragrans ORCHID
Thelymitra ixioides subsp. ixioides ORCHID
Thelymitra malvina ORCHID
Thelymitra pauciflora ORCHID
Thelymitra purpurata ORCHID
Themeda australis NT
Themeda triandra NT
Thespesia populneoides NT
Thryptomene baeckeacea NT
Thryptomene costata NT
Thryptomene denticulate NT
Thryptomene lancelin NT
Thryptomene saxicola NT
Thryptomene urceolaris NT
Thysanotus juncifolius NT, SA
Thysanotus tuberosus subsp. tuberosus NT, SA
Tinospora smilacina NT
Tmesipteris obliqua FERN
Tmesipteris ovata FERN
Tmesipteris parva FERN
Tmesipteris truncata FERN
Todea barbara FERN
Toechima dasyrrhache NT
Toechima tenax NT
Toona ciliate NT
Trachymene composite NT, STVC
Trachymene incise NT, STVC
Trachymene procumbens NT, STVC
Tragia novae-hollandiae NT
Trema tomentosa sub sp. aspera NT
Trema tomentosa ub sp. tomentosa. NT
Trichodesma zeylanicum NT
Trichosanthus cucumerina STVC
Trichosanthus subvelutina STVC
Tricoryne elatior STVC
Tricostularia pauciflora NT
Triglochin multifructa BM
Triglochin procera BM
Triglochin striata BM
Trimenia moorei NT
Tripladenia cunninghamii NT
Triplarina imbricata NT
Tristaniopsis collina NT
Tristaniopsis laurina NT
Triunia youngiana NT
Trochocarpa laurina NT
Trochocarpa montana NT, STF
Trophis scandens subsp. scandens NT
Turraea pubescens NT
Tylophora barbata NT
Tylophora benthamii NT
Tylophora grandiflora NT
Tylophora woollsii NT
Typha orientalis BM
Typhonium brownii NT
Uraria lagopodioides HW
Uromyrtus australis NT
Uromyrtus lamingtonensis NT
Urtica incisa NT
Utricularia aurea SUB
Utricularia australis SUB
Utricularia biloba BM
Utricularia caerulea BM
Utricularia dichotoma BM
Utricularia gibba SUB
Utricularia lateriflora BM
Utricularia uliginosa BM
Utricularia uniflora BM
Uvaria concava NT
Uvaria leichhardti NT
Vachellia farnesiana BW
Vallisneria australis SUB
Vallisneria nana SUB
Vappodes phalaenopsis ORCHID
Velleia paradoxa NT
Velleia spathulata NT
Verbena gaudichaudii NT
Vernonia cinerea NT
Veronica grosseserrata NT
Veronica plebeian NT
Verticordia auriculata NT
Verticordia brownii NT
Verticordia grandis NT
Verticordia mitchelliana NT
Verticordia nitens NT
Verticordia plumosa NT
Vesselowskya rubifolia NT
Vesselowskya venusta NT
Vigna luteola NT
Vigna marina HW, SA
Vigna vexillata subsp. angustifolia HW, SA
Vigna vexillata subsp. marina HW, SA
Vigna vexillata subsp. youngiana HW, SA
Viminaria juncea HW
Viola banksii DIV
Viola betonicifolia NT
Viola caleyana DIV
Viola hederacea DIV
Viola silicestris DIV
Viscum articulatum NT
Vitex melicopea SA
Vitex rotundifolia SA
Vitex trifolia SA
Vittadinia hispidula subsp. hispidula NT
Vittadinia hispidula subsp. setosa NT
Vittaria elongata FERN
Wahlenbergia communis STVC
Wahlenbergia gloriosa STF
Wahlenbergia gracilis STVC
Wahlenbergia stricta STVC
Waterhousea floribunda NT
Waterhousea unipunctata NT
Wedelia spilanthoides NT, BM
Westringia amabilis NT
Westringea cephalantha NT
Westringea fruiticosa NT
Westringia glabra STVC
Westringea longifolia NT
Westringia sericea NT
Wikstroemia indica SA
Wilkea austroqueenslandica SA
Wilkea huegeliana SA
Wilkea macrophylla SA
Willdampia formosa SA
Wodyetia bifurcate SA
Wolffia angusta DIV
Woollsia pungens SA
Wrightia laevis NT
Wrightia pubescens NT
Wrightia saligna NT
Wurmbea biglandulosa NT
Xanthophyllum fragrans NT
Xanthophyllum octandrum NT
Xanthorrhoea fulva NT, SA
Xanthorrhoea glauca subsp. glauca NT, SA
Xanthorrhoea johnsonii NT, SA
Xanthorrhoea latifolia subsp. latifolia NT, SA
Xanthorrhoea latifolia subsp. maxima NT, SA
Xanthorrhoea macronema NT, SA
Xanthorrhoea malacophylla NT, SA
Xanthosia pilosa NT
Xanthosia tomentosa NT
Xanthostemon chrysanthus NT
Xanthostemon paradoxus NT
Xanthostemon whitei NT
Xanthostemon youngii Veins NT
Xerochrysum bracteatum NT
Xerochrysum viscosum NT
Xylosma terraereginea NT
Xyris bracteata BM
Xyris complanata BM
Xyris gracilis BM
Xyris juncea BM
Xyris operculata BM
Youngia japonica NT
Zanthoxylum brachyacanthum NT
Zeuxine oblonga ORCHID
Zieria arborescens subsp. arborescens NT
Zieria arborescens subsp. deccurens NT
Zieria compacta NT
Zieria furfuracea NT
Zieria laevigata NT
Zieria laxiflora NT
Zieria minutiflora NT
Zieria prostrata NT
Zieria smithii NT
Zieria southwellii NT
Ziziphus oenopolia Flowering NT
Zornia dyctiocarpa NT
Zornia floribunda NT
Zornia muriculata NT
Zoysia macrantha NT
Cuttings
Many vegetative parts of a plant can be used for propagation of new plants. The most common methods of propagation consist of Stem cuttings, root cuttings, eye cuttings and leaf cuttings. Cuttings offer the gardener the chance to grow plants that do not readily set seed; seed is often sterile or difficult to germinate under nursery conditions. Cutting grown plants exhibit exactly the same DNA traits as the parent plant. Cutting grown plants gives you the opportunity to select the desired characteristics you desire in height, shape leaf or flower colour, vigor or maybe salt tolerances.
The potting mix for cuttings has to be sterile, low in fertility, and well-drained to provide sufficient aeration yet have good moisture retention, so that watering does not have to be done too frequently. Materials commonly used are one part coarse sand, one part peat and one part perlite (by volume), or one part peat and one part coarse sand (by volume) or two parts sand and one part vermiculite (by volume). The mix should be well watered and allowed to drain prior to preparation of the cuttings.
- Stem cuttings are the most common method. This is where a section of the stem is partly buried in the soil, including at least one leaf node. The cutting is able to produce new roots, usually at the node. Stem cuttings are divided into soft wood, semi hardwood and hardwood cuttings.
As a general guide the following seasonal wheel may help in deciding what is the best time of the year different types of cuttings should be taken. Soft wood and semi hardwood cuttings can be extended if frosts are not a problem and the early spring and late autumn weather is warm.
Softwood or Herbaceous Cuttings: These are the easiest type of plants to propagate from. Use 30mm to 70mm long herbaceous cuttings taken from September to late January or as late as March if you are in a frost free area. Vigorously growing plants of the form you desire should be used when growing from cuttings Place the cuttings in a cool semi shaded position preferably under 30mm to 70mm shade cloth.
Semi Hardwood Cuttings: These are a little more difficult to grow and take longer to strike. Use 40mm to 90mm long semi hardwood cuttings taken from January to mid June or as late as mid July if you are in a frost free area.
Hardwood Cuttings: Hardwood cuttings are taken from dormant, mature evergreen stems or deciduous plants. Use 70mm to 150mm long hardwood cuttings taken from late February to mid early September or as late as early October in the south where dormancy prevails longer.
The three types of hardwood cuttings are straight, mallet and heel. A straight cutting is the most commonly used stem cutting. Mallet and heel cuttings are used for plants that might otherwise be more difficult to root. For the heel cutting, a small section of older wood is included at the base of the cutting. For the mallet cutting apart of the main branch or stem is included at the base of the cutting stem.
Procedures for Stem Cuttings:
As the procedure is similar for each type of cutting I will discuss the cuttings as one group with specific mentions where they vary. The following instructions are a general rule and local conditions with the individual plant requirements must be considered.
Plants selected for cuttings must be healthy, free of disease and preferably from the upper or outer sections.
The health of the stock plant is vitally important for success. If possible avoid plants with symptoms of mineral or nutrient deficiencies. Also avoid plants that have been fertilized in the past 6 to 8 weeks particularly those with high nitrogenous soft foliage as they will not root well. The stock plant should not be under moisture stress.
Generally speaking younger plants root better than their older, more mature cousins. Lateral shoots often root better than cuttings from terminal shoots. Lateral shoots can be induced by tip pruning 5 to 8 weeks prior to taking the cuttings.
Early morning is the best time to remove the material from the stock plant. Once the material has been removed it is important to keep the cuttings cool and moist until the cutting is prepared and placed in the green house. Black plastic bag with wet paper towels may be used to store cuttings. Try to avoid any delays from the time the material is removed from the stock plant to the final preparation.
The present season or past season’s growth is the most suitable. Avoid material with flower buds if possible. If it is possible take the cuttings a month earlier or after flowering has ceased. If they cannot be avoided then remove all the flowers and buds as you prepare the cuttings. This allows all the cuttings reserves or energy to be used in producing new roots.
The material size depends on the genus, species and type of cutting material being used. See Above. Use a sharp, stainless steel, thin bladed pocket knife that has been sterilized in alcohol. Continually dip the cutting tool in the alcohol to prevent transmitting diseases from infected plant parts to healthy ones.
First make a diagonal cut at the base on a 45 degree angle just below the node. Then remove the leaves from the lower one third to one half of the selected cutting. Plants with large leaves may need the apical half removed to reduce water loss and conserve space.
Species difficult to root should be wounded near the base. Wounding a stem refers to slightly crushing or damaging the base of the stem. Another method is to remove a small section of the bark from the base on one side. Be gentle. The diagram below right shows a single node cutting with the 45 degree cut made below the node.
Root hormones can be a valuable resource to help stimulate root development. This is generally not required on herbaceous or soft wood cuttings but is more important on hardwood and difficult to strike plant material.
To prevent contamination of the hormone place a small amount on the surface of another container. I prefer to use the lids off soft drink bottles. Hormone that is not used should be discarded as rubbish. Be sure to tap the cuttings to remove excess hormone when using a powder formulation. Liquid hormones are generally easier and more reliable to use as there is less wastage and the amount used on each cutting can be controlled.
Insert the cuttings using a dipple stick do not force the cutting into the mix. Insert it to a depth of one-third to one-half of its length into the medium. Maintain the vertical position with the apical end to the top. Make sure the buds are pointed up. Space cuttings far enough apart to allow all leaves to receive sunlight. Gently water again with clean sterile water after each tray or pot has been filled. Cover the cuttings with plastic bag and place in indirect light beneath 30mm to 70mm shade in a warm position. Keep the medium moist until the cuttings have rooted. Rooting will be improved if the cuttings are misted on a regular basis if not covered in plastic. I use old icecream sticks for dipple sticks and long meat skewers with taped on one end for the posts so that the plastic does not fall down onto the cuttings.
Rooting times vary greatly with the type of cutting, the genus and species being rooted and environmental conditions. Once rooted, they may be left in the rooting structure until spring.
Root cuttings
Root cuttings can be used on plants that have surface roots or long thick rhizomatosous roots. The cutting is taken where a section of root is buried just below the soil surface. This section produces new shoots from root nodes. Take 25mm to 75mm sections of roots and place the cuttings horizontally about 8mm to 10mm below the soil surface. This method is usually used indoors or in a hotbed. Many of the Homalanthus specie and Melaleuca specie reshoot readily using this method as do many creeping grasses.
Eye cuttings and heal cuttings
These cuttings are used when cutting material is in short supply. The eye refers to the bud which emerges from the leaf axils at each node.
Single Eye: This is used for plants with alternate leaves when space or stock material is limited. Cut the stem 8mm to 10mm above and below a node. Place the cutting on a 45 degree angle in the medium with the node just touching the surface. The leaf most probably will need support. I use a meat skewer and carefully place it through the leaf and into the soil for support.
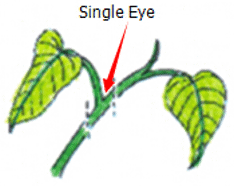
Double Eye: This is used for plants with opposite leaves when space or stock material is limited. Cut the stem about 8mm to 10mm above and below the pair of nodes. Insert the cutting vertically in the medium with the node just touching the surface of the medium. The leaves can lie on the mix. The leaves of larger leaf plants will need to be trimmed.
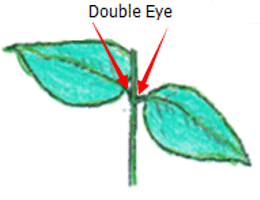
Leaf cuttings
Leaf cuttings use different parts of a leaf where the leaf is placed on a moist sterile mix. These have to develop both new stems and new roots. Some leaves will produce one plant at the base of the leaf. In some species, multiple new plants can be produced at many places on one leaf, and these can be induced by cutting the lateral veins
Leaf cuttings are used almost exclusively for a few indoor plants. Leaves of most plants will either produce a few roots but no plant, or just decay.
Whole leaf with petiole: Detach the leaf with the petiole in tact. Insert the petiole to the leaf base into the medium. One or more new plants will form along the petiole. It is more often used on plants with fleshy leaves. Asplenium bulbiferum can be propagated using this method.
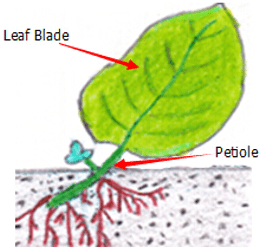
Whole leaf without petiole: This is used for plants with sessile leaves. Insert the cutting vertically into the medium. A new plant will form from the axillary bud. It is more often used on plants with succulent type leaves Viola betonicifolia can e reproduced using this method..
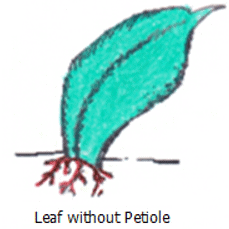
Split vein: Detach a leaf from the stock plant. Slit its veins on the lower leaf surface being careful not to sever the vein completely. Lay the cutting, lower side down, on the medium. The petiole can be used to help secure the leaf so that it does not move. New plants will form at each cut. If the leaf tends to curl up, hold it in place by covering the margins with the rooting medium. Carpobrotus species and probably most other native succulents would propagate using this method.
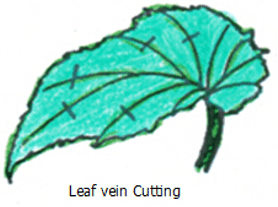
Leaf sections: This method is frequently used with snake plant and fibrous rooted begonias. Detach the leaf and cut the leaves into wedges with at least one main lateral vein. Lay leaves flat on the medium. A new plant will arise at the vein. I am not aware of any Australian Native plants where this method has been used on a regular basis.

Runners
Plant runners are a way in which some plants can be propagated asexually. A plant runner is a rapidly growing stem that grows above the ground and on the soil surface. The stock plant usually produces a set number of runners which eventually, produces new individuals from the tips of their nodes. As the new plant is slowly developing, the stems of the plant continue to take advantage of the rich soil and grow, while the roots begin to get stronger and support the newly created offspring. These can be severed and placed in a pot, watered and maintained for a few weeks until the new roots have fully developed. Viola hederacea, Pratia specie, Dichondra repens or Lastreopsis specie are all easily propagated using this method.
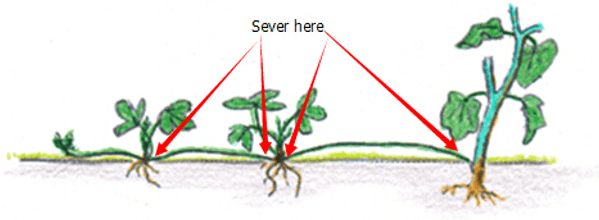
Division
First select a healthy plant that is disease free. It is best to divide plants just prior to the onset of new growth. Prior to removing the plant from the ground plants should be given a deep watering a few days before removal, then again about 2 hours before removing them from the ground. It is best to soak potted plants in water for a couple of hours prior to removing the plant from the pot.
Use a spade and cut down each side about 100mm out from the base of the plant. Dig back on one side so that the spade can be wedged below the plant. Now lift the plant out trying not to damage the roots.
Wash the roots gently but thoroughly to remove all the soil.
Divide the clumps so that there is a crown or tuff with roots and leaves on each division. This is best done using secateurs, a large knife or spade in very large clumps.
Remove any unwanted or dead roots.
Remove half the foliage leaving those leaves that surround the growing point.
Spread the remaining roots outwards and downwards to allow the plants the maximum room to develop new roots.
Water thoroughly but do not fertilize. Place the containers beneath 30mm shade cloth and keep moist. After a fortnight or after the signs of new roots or shoots fertilize with our recommended fertilizer and repeat after about a month.
Once the plants have re-established themselves plant out and fertilize as before.
Division is a term applied to a number of propagation methods where by a plant is propagated by splitting it into 2 or more pieces all of which have roots stems, culms or stolons. Division is most often used with plants which form grass-like clumps which include Rushes like Eleocharis acuta, lilies like Anigozanthos flavida or even some larger type ferns like Acrostichum specie.
Grass-like clumps
All Grasses and Reeds that are declared rare, vulnerable or endangered are protected by Federal and State Laws and must not be removed from the wild unless you are a land developer, mining company or main Roads department etc. This includes bulbs, roots, leaves and flowers. No part of any plant can be removed from Federal, State or Local Government land without the prior permission of the authority.
“Grassy clumps” generally have strap-like leaves arising from an underground rhizome which is a thickened underground stem. The best known Australian plants in this group are the Lomandra specie or Juncea specie. Less well known are the Dianella specie and Stypandra specie both with spikes of small blue flowers.
Water the plants well a day or 2 before the operation is to be done. Avoid very hot weather. Cut around the clump and out on one side so that the shovel can be placed under the root ball.
Remove the plant from the soil. Cut it into 3 or 4 equal parts, first down the middle then halve those sections again ensuring that each section has some roots and, rhizome and culms or shoots. The rhizomes should have at least several eyes or growing shoots. Remove unwanted dead leaves and any old small sections that look weak or dead. Plants can be divided further but ensure each division has a several strong shoots and healthy roots attached to the tuff. Replant ensuring the soil is at the same level as before. Water and fertilize. New shoots will appear within two weeks.
Divided into segments so that each one comprise a piece of the rhizome, some leaf shoots and some roots. Dead leaves and dead roots should be removed. If you just want a few plants for your own use, the best sized division comprises three or four leaf shoots with a good section of rhizome and healthy roots. With these, the humid environment of a greenhouse or similar shelter is probably not required, just a sheltered position and regular watering.
If a humid greenhouse environment is available, the divisions can be quite small, comprising only one set of leaf shoots. Once potted into individual small pots, these are kept in the greenhouse for a few weeks (4 to 6 weeks in the warm part of the year; longer at other times). It may be beneficial to trim the leaves to reduce moisture loss through transpiration. Divisions of this size will also need to be “hardened” after the roots have developed. A simple method is to expose them to the open air during the night for a week or so, putting them back into the humid shelter during the day. After this period they can be moved to an outdoor, sheltered position for at least another week before potting on.
Suckers
There are a host of plants which spread by producing suckers from their root systems. Some of these are so vigorous and invasive that propagation is probably the last thing on the gardener’s mind. Rubus specie, especially the exotic Rubus fruiticosus is well known for its voracious suckering habit. Others, however, are less anti-social.
Among the Australian natives Dampiera species and the related Lechenaultia species often spread by suckering as do some Lobelia specie, Scaevola specie and Peperomia specie. Even seemingly unlikely genera such as Grevillea and Acacia have some specie that sucker.
All you have to do with this group is to dig up small pieces, make sure that there are roots attached and repot them into individual containers. Water them with our recommended fertilizer and place in a sheltered position beneath 30mm to 50mm shade cloth for a few weeks.
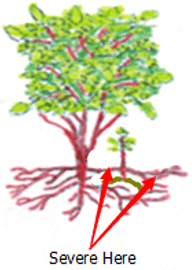
Layering
Layering is a propagation technique which is not used all that often these days, where a branch of a shrub is pegged down, covered with soil and then severed from the parent plant when roots have formed. There are times when nature does all of the work for us. This method is useful for propagating small numbers of plants that are relatively difficult to propagate by any other method. I have grown Eucalyptus specie, Mallotus specie and Eleocarpus using this method just for fun. Plants are usually well advanced with the new plants being several meters in height.
Most of these “self-lay” and are ground covers where branches are in contact with the ground due to the growth habit of the plants. Several Hibbertia specie are known to form colonies by layering. Hibbertia pedunculata often form roots at many points along its stems especially in warm humid climates. Propagation is simply a matter of damaging the stem between the section required and the stock plant. Once it has developed roots sever the stem into one or more sections provided each contains some roots and treat them similar to rooted cuttings. It is best done in the early morning and avoid hot weather.
Another group of Australian plants are the Ficus species, Conostylis species and Myoporum specie which all develop lateral stolons above the ground which root at the leaf nodes. These usually form small plantlets where they touch the ground and these can be cut off and potted up.
If you don’t have access to a greenhouse you can still successfully propagate layered species in your garden. Just place a small pot of mix under the branch and place the roots into it. If necessary, peg the branch down with some wire to prevent the roots being pulled out of the mix by the wind. After a few weeks the roots should be well established in the mix and the new plant can be severed from its parent. An easy way to grow ground covers.
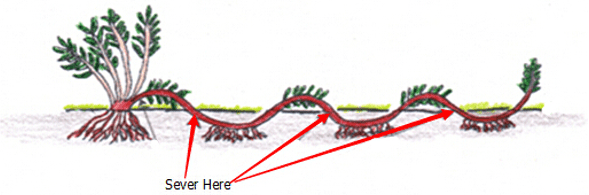
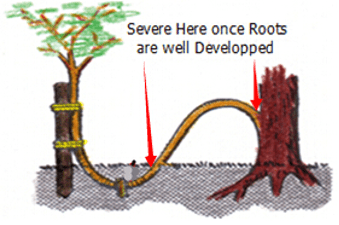
The branch needs to be secured. Instead of the ground a large pot can be used. Damage the stem on the lower surface just before or as it touches the ground or immediately after the stake. Another method instead of a pot is to securely wrap the wound in moist peat, or sphagnum moss and seal with a weather proof tape. Once roots or new shoots appear the new cutting can be severed below the tape and gently repotted.
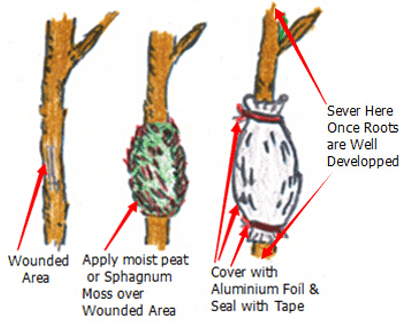
Corms, Tubers & Rhizomes
All Corms, Tubers & Rhizomes that are declared rare, vulnerable or endangered are protected by Federal and State Laws and must not be removed from the wild unless you are a land developer, mining company or main Roads department etc. This includes bulbs, roots, leaves and flowers. No part of any plant can be removed from Federal, State or Local Government land without the prior permission of the authority.
For the serious grower temperatures are best if they are between 20 degrees centigrade and 32 degrees centigrade during the growing season and 2 degrees and 8 degrees when the tubers are maturing and hardening off.
The ground needs to be relatively level and well prepared to a depth of 300mm. Light fatty clay is preferred by most bulbous plants. A rich layer of well composted animal manure and plant waste composted at 50:50 ratio is ideal. Add a light spreading of Hood’s Blood and Bone so that the phosphorous levels are adequate to grow good tubers. Lime should be added to bring the pH into 6.5pH to 7.2pH depending on the genus and specie to be grown. The compost should be about 20mm thick and lightly turned into the soil with the lime and blood and bone.
Plant the corms in early spring after the last frosts have abated. Plant them at 450mm to 500mm centers with the rows at 500mm apart. Sow the corms at a depth of 50mm. Do not plant any closer as this will cause a sever decrease in the number of corms, tubers or rhizomes set by the plants in autumn.
Water and keep the soil moist to allow growth to reach around 100mm in height without setting the plants back. It is important to maintain soil moisture for the rest of the growing season which should be around 6 to 7 months. In late autumn, when the blooms have peaked water can be withdrawn.
Once the plants dye down the corms, tubers or rhizomes can be retrieved and stored for the winter. Most corms, tubers or rhizomes wil require a period of dormancy in the vegetable crisper over winter before sowing the following year.
With Water Chestnuts the soil should be flooded once the plants reach 100mm to 120mm in height to a depth of 30mm to 80mm in depth. In autumn after the plants begin to brown off at the tips the water can be naturally drained or evaporated completely. It is now that the hardening off of the chestnuts will commence. If rainfalls refill the area then it must be drained immediately. Leave the plants for 3 to 5 weeks to mature properly corms should be a rich chestnut brown in colour when they are ready for harvesting.
A similar process can be done using seeds to attain a large number of smaller corms which can be sown the following year.
Bulbs: New bulbs form beside the originally planted bulb. Separate these bulb clumps every 1 to 3 years to maintain the largest blooms and to increase bulb numbers. Dig up the clump after the leaves have withered. Gently pull the bulbs apart and store them in a cool dry place for 2 to 4 days before replanting them. Small, new bulbs may not flower for 2 or 3 years, but large ones should bloom the first year.
Corms: A large new corm forms on top of the old corm, and tiny cormels form around the large corm. After the leaves wither, dig the corms up and allow them to dry in indirect light for 2 or 3 weeks. Remove the cormels, then gently separate the new corm from the old corm. Dust all new corms with a fungicide and store in a cool place until planting time. Place the corms in the vegetable crisper 2 to 3 weeks prior to sowing. This will ensure all the corms sprout together and flower at the same time.

Rhizomes: New rhizomes form as an extension of the old rhizome. Dig the rhizomes up when the plants look ragged and unkempt which is usually during the early winter months. Separate the rhizomes and sever mature rhizomes ensuring every clump has several eyes. Cut the leaves off just above the old ground level. Replant at the old level or just below the surface in the newly rejuvenated beds and water thoroughly with our recommended fertilizer. the native ginger Alpinia caerulea or Alocasia brisbaniensis can be reproduced using this method..
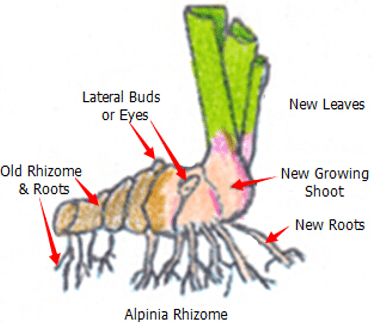
Grafting
The three methods which I have found successful on the orchard for citrus are bud grafting wedge grafting and for smaller numbers the approach graft.
Bud grafting is suitable where large numbers of plants are need to be done, the material is of two different sizes or if the parent scion plant is in very limited supply. The stock plant is the plant with the roots. The scion is the piece of material that is required to fruit or bloom at a later date and is at the top.
Equipment needed are grafting tape ( A good stretching grafting tape not a cheap one that does not stretch or breaks easily), sharp razor blade knife, grafting mastic or grafting plasticine and sterilizing solution.
The Cleft and wedge grafts are the most commonly used on orchards and has the advantage of being easy and quick to accomplished with larger trees bearing fruit sooner.
The bud graft is used where the scion material is in limited supply or very large numbers are required. The bud usually takes quickly but may be 6 months behind the cleft and wedge graft meaning a full season is lost in fruit production.
The approach graft is seldom used and is used for small numbers. It has a high success rate with larger trees being grown in the first year. It is the ideal method for home gardeners.
Cleft or Wedge Graft:
- The stock plant is cut off at 50mm to 100mm above the collar making sure that there are a few leaves left in tack but not within 20mm of the cut.
- The scion is selected so that it is 60mm to 90mm long and the base is the roughly same diameter as the stock.
- The stock plant now has a 5mm to 15mm VEE notch cut into it with the knife after the blade has been sterilized. DO NOT TOUCH THE NEW CUT WITH THE FINGERS. Contaminants and oil may affect the bond.
Cut the base of the scion back 5mm to 15mm to the same shape as the notch on the stock so that it fits tight. DO NOT TOUCH THE NEW CUT WITH THE FINGERS. Contamina
- Contaminants and oil will affect the bond.
- Insert the scion into the notch of the stock so that the xylem are in contact.
- Wrap the grafting tape around the stock and scion starting from the bottom and moving to the top of the graft and back down again. Tie the tape off. Start about 10mm to 15mm below the graft and finish 10mm to 15mm above the graft.
- Apply grafting mastic, plasticine over the tape to ensure water does not enter the site.
- The graft if successful will see the scion shoot new growth in 4 to 6 weeks. There is no need to remove the tape if a good quality one is used as it will expand as the union takes and will eventually split and drop off.
Bud Graft:
- The stock plant is cut off at 200mm to 300mm above the collar making sure that there are a few leaves left in tack near the top of the plant.
- The scion is selected as a new developing bud that has begun to swell.
- The stock plant now has a 10mm to 15mm VERTICAL SLIT at around 100mm above the collar with the sterilized knife blade. DO NOT TOUCH THE NEW CUT WITH THE FINGERS. Contaminants and oil may affect the bond.
- Remove the bud from the growing tree so that there is a 10mm heel left on it. DO NOT TOUCH THE NEW CUT AREA WITH THE FINGERS. Contaminants and oil may affect the bond.
- Insert the bud into slit made in the stock.
- Wrap the grafting tape around the stock and scion starting from the bottom and moving to the top of the graft and back down again. Tie the tape off. Ensure there is a gap where the bud can be seen and can emerge. Start about 10mm to 15mm below the graft and finish 10mm to 15mm above the graft.
- Apply grafting mastic, plasticine over the tape to ensure water does not enter the site.
- The graft if successful will see the bud shoot new growth in 3 to 4 weeks. There is no need to remove the tape if a good quality one is used as it will expand as the union takes and will eventually split.
- Cut the top off the stock plant at a 45 degree angle starting at the bud and going upwards towards the apex and away from the bud. This will leave an area of bare wood where the new shoot will eventually cover.
Approach Grafting:
- The stock plant is allowed to grow normally in a pot to 300mm to 500mm tall. Trim leaves off at the area where the union will take place.
- The scion is also allowed to grow in a pot to 300mm to 350mm tall or can be an existing tree on the block. Trim leaves off at the area where the union will take place.
- Remove the top of the stock plant abot 50mm above the area of where the union will take place.
- The stock plant now has a 10mm to 15mm slice removed from one side of the stem or trunk with the knife after the blade has been sterilized. DO NOT TOUCH THE NEW CUT WITH THE FINGERS. Contaminants and oil may affect the bond.
- The scion plant now has a 10mm to 15mm slice removed from one side of the stem or trunk with the knife after the blade has been sterilized. Make sure the cuts align before you cut the scion. DO NOT TOUCH THE NEW CUT WITH THE FINGERS. Contaminants and oil may affect the bond.
5. Bring the scion and stock together so that they touch and can be taped together tightly. If this is done out doors make sure the two sections are stable and won’t get blown about and kill the union.
- Wrap the grafting tape around the stock and scion starting from the bottom and moving to the top of the graft and back down again. Tie the tape off. Start about 15mm to 25mm below the graft and finish 15mm to 25mm above the graft.
- Apply grafting mastic, plasticine over the tape to ensure water does not enter the site.
- The graft if successful will see the scion shoot new growth in 4 to 6 weeks. There is no need to remove the tape if a good quality one is used as it will expand as the union takes and will eventually split.
- Make a cut below the graft on the scion so that it is no longer reliant upon its own roots. Make this cut as close as possible to the union using a sterile sharp pair of secateurs. Seal the wound with grafting wax. Be careful not to damage the union.
- Remove by trimming the stock plant back as close as possible to the union with a sterilized pair of sharp secateurs. Seal the wound with grafting wax. Be careful not to damage the union.
Fern Spores:
All ferns that are declared rare, vulnerable or endangered are protected by Federal and State Laws and must not be removed from the wild unless you are a land developer, mining company or main Roads department etc. This includes bulbs, roots, leaves and flowers. No part of any plant can be removed from Federal, State or Local Government land without the prior permission of the authority and this includes the spore.
While ferns do not flower they do however produce sexually, producing an egg and sperm. the sperm are reliant on weather conditions to swim from their antheridium to the egg’s archegonium to complete the process of fertilization. (For further details and explanations see “The Science of Botany chapter 14 Fern reproduction”)

Most people are put off at the thought of growing ferns from spore. Like all plants that produce their offspring from seed or spore the methods are basically the same. Remember nature has been doing this for millions of years and has been very successful. I have had excellent results growing over 260 different species of Australian ferns so give it a go.
Step 1. Selecting mature spore from the fern fronds, which has just started to release its spore. Rinse the fronds under clean running water and dry. This is to wash off any other spores from rogue ferns that may have settled onto the fronds. (There is nothing worse than having common brake or common bracken contaminating a prized tree fern or epiphyte.)
Step 2. Place the dry fronds in a clean brown paper bag and keep them in a cool dark place like the linen closet for a few days to a week before you are ready to sow the spore.
Step 3. Take a large ice cream container, a small ice cream container and a clean clear plastic bag large enough to seal the large ice cream container.
Step 4. Wash both containers so that they are very clean and sterile.
Step 5. Punch or drill some small holes in the bottom of the small ice cream container.
Step 6. Use a clean fine seed raising mix that has had some clay or crusher dust added to it. About 10mm is great or add around 30mm peat. Then moisten the mix. epiphytic ferns do better on the peat than clay.
Step 7. Moisten and place the mix in the microwave oven with a large glass of water for 7 or 8 minutes, until the water is boiling. Allow them to cool in the oven. You will need the water later so do not tip it out.
Step 8. Take the brown paper bag out of the linen closet. Shake the bag and remove the fronds. You should have a brown, fawn powder, small round pepper size spore or yellow green spore depending on the specie involved.
Step 9. Remove the mixture from the oven once it has cooled and place it in the small ice cream container.
Step 10. Sprinkle the spore sparsely over the mixture in the small ice cream container.
Step 11. Place the small ice cream container in the large ice cream container.
Step 12. Remove the water from the microwave and pour it into the larger ice cream container so that there is 20mm to 25mm of water in the bottom.
Step 13. Place the ice cream containers in the plastic bag and seal.
Step 14. Place the contents and bag in a warm shady place preferably 50mm to 70mm shade depending on the specie. Shade houses and some window sills are ideal.
Step 15. Wait until the ferns are 15mm to 25mm high before you attempt to transplant them. Once they are ready open the bag up slightly and allow the air to flow around the little ferns. Every 3 to 5 days open the bag a little further so the ferns get use to their new environment. Allow them a week or two to harden off before you transplant them into 50mm standard tubes.
Do not try to transplant them as single plants as they are a little delicate still.
Platycerum and Asplenium will have to be divided into single plants. Be careful as they are still fragile at this stage. Replace them close to their original position. After a few weeks the ferns will power away without any set backs. Here they can be further divided where necessary removing the smaller ferns leaving the largest one in place.
Once they reach 50mm to 60mm you may wish to divide the stronger and hardier individual plants into smaller clumps in 100mm squat pots. The closer you mimic the wilderness areas where the ferns grow the better your results. Remember not all ferns like Polystitchum australe grow in lush rainforest conditions – Platycerum prefer the brighter outerlimits of branches even growing on Avicinea species or mangroves in days past while Drynaria grow on exosed rocks where drier and harsher conditions prevail and Lindsea are often found on poorer baren clays in dry, open, schlerophyll forests.
Step 16. Fertilize using Seaweed, fish emulsion or organic chicken pellets soaked in water on an alternate basis. Fertilize every two months for one year even when in the ground then follow up on an annual basis in September or early March.

ORCHIDS
All orchids are protected by Federal and State Laws and must not be removed from the wild unless you are a land developer, mining company or main Roads department etc. This includes bulbs, roots, leaves and flowers. No part of any plant can be removed from Federal, State or Local Government land without the prior permission of the authority and this includes the seeds.
Division: is the easiest method for amateurs to propagate Dendrobium and many other epiphytic orchids. At some point, the orchid will become root bound in its pot or large enough to divide the clump on a tree or slab. The only tools you require is a sharp sterile knife a slab of cork or pumice stone and some hemp or nylon string. I prefer hemp string because it is totally natural but is difficult to obtain.
The potted Dendrobium: Remove the Dendrobium from its pot and set it on a firm surface. Cut through the roots to create separate sections of the plant. Each piece should include four to five stems and a large chunk of the root system. Repot using a central stick to ensure the plant is tied securely so that it does not move.
Slab plants: Take the slab and cut it down the middle ensuring each section has healthy terminal stems. Retie the new divisions back to the new piece of cork or pumice stone. Tie them so that they do not move. Do not over tighten as this cut’s off the flow of nutrients to the new leaves.
Treat plants that are growing naturally on a tree in the same way. First cut the plant in half then slice just below the roots into the outer layer of bark being careful not to damage the stems or the xylem and phloem layers of the tree. Remove the section and retie it to another section of the tree or cork slab as desired.
Back Bulbs:
Back bulbs can be taken from mature plants.
- Remove the parent plant from its container.
- Divide the plant up so each new plant has an old back bulb without leaves, an old bulb with leaves and maybe a new bulb that hasn’t flowered or isn’t fully developed.
- Remove or straighten the roots out and cut so that they are around 100mm long. Remove all the roots from the old back bulb without leaves.
- Allow to air dry for 24 hours to 48 hours.
- Repot into a suitable size pot using an orchid mix or tie firmly so that they do not move before rooting into the fork of a new tree.
If the plant is existing in your garden and you wish to use this method of propagating choose a day that follows a week of cool weather. Select the division as above and slice through the plant behind an old bulb without leaves, ensuring the new plant has an old bulb with leaves and a new bulb that has not fully developed. Repeat as above in steps 3 to 5.
Seed:
Orchid seeds require treatment before sowing. Really Orchid seeds are meant for professionals with time, equipment and space. However if you wish to persevere then here are the basics and you are learning from an amateur. You will need the following flask, Chloros solution, boiled water, an eye dropper, paper bag, a warm sheltered position and some patience and luck.
- Collecting the seed: Seed can only be collected from fully ripe pods. You may need to watch a pod for several days or even weeks following the time it stops swelling for it to turn yellow. At this stage the pod must be watched carefully for the pod to commence spitting along the placenta. If possible place the paper bag over the pod and tie it off. If necessary place a plastic bag over the paper bag to prevent rain or moisture contacting the seeds.
- Knowledge is very important now, as different species and genre have various numbers of viable seed in a pod. Viable seeds may be as few as 20 or as many as several million. Check the bag daily and remove once the seed begins to disperse. Shake all the seeds from the pod into the bag.
- Purchase the appropriate agar solution from an orchid society as they will know what you want and which one will be best for your species. Prepare the agar solution and pour into the flask.
- Remove some of the seeds by shaking them into a solution of 1 part chloros to 20 parts water and allow soaking for 10 minutes.
- Remove the seeds with the eye dropper in the solution and spread evenly over the set agar.
- Place the flasks in a room that is will lit with indirect light and the temperature is a consistent 25 to 30 degrees centigrade.
- Wait 4 to 7 months for the seedlings to develop to a stage where they can be removed and planted on into a fine orchid mix. Do not remove the seedlings from their environment of light or temperature for at least another two months. Even at this stage it is best to place the seedlings in a green house where their environment can be controlled.
Seed should be shaken in Chloros (usually 1 in 20 with water) for 10 minutes before planting to kill any disease. Sow using droppers or needles in a sterile environment, into flasks. Seal and place in high humidity, warm environment. It normally takes 4 to 6 months to raise seedlings to the point where they can be transplanted. It can take 4 to 10 years for the seedlings to flower
Terrestrial Orchid Cultivation
All orchids are protected by Federal and State Laws and must not be removed from the wild unless you are a land developer, mining company or main Roads department etc. This includes bulbs, roots, leaves and flowers. No part of any plant can be removed from Federal, State or Local Government land without the prior permission of the authority.
Native terrestrial orchids comprise of many Genus with their individual specie which range from being relatively easy to cultivate to those that are damn near impossible and definitely frustrating to say the least.
Most terrestrial orchids are deciduous during the dry season. These usually commence their growing season with the onset of the wet season through to the end of the wet season. The flowering occurs shortly after the leaves have been established sending up the new scape flowering then dying back to a subterranean tuber in a yearly cycle of growth.
The more commonly grown genre in Australian cultivation include the Acianthus, Caladenia, Caleana, Diuris, Pterostylis and Thelymitra. It is for this reason I will concentrate on these genre which have proven reliable by many enthusiasts. Many other native, terrestrial Genre not included may still be cultivated under these guidelines with decreased successes until a lot more research has been completed.
The saprophytic orchids have been deliberately excluded because they are highly specialized orchids in nature that replicating their growing conditions is near impossible for any period of time. This intriguing group of orchids has some of the most amazing blooms of any orchid with the above mentioned groups worthwhile persevering with.
From the delicate Caladenia species (Spider Orchids), the amazing Caleana species (Duck Orchids), the brilliantly coloured Diuris species (Donkey Orchids), the curious Pterostylis species (Greenhoods) and the spectacular Thelymitras species (Sun Orchids) ground orchids are just beginning to charm the gardener. These orchids have some of the most amazing colour combinations and shapes of any flowers found anywhere in the world.
The life cycle needs to be fully understood prior to any attempt to grow these beautiful orchids or failure is assured. The best advice I can give is if you are lucky enough to have these orchids growing naturally preserve their environment as nature has done for millions of years. I marked all the active growth areas on my Nana Glen property with survey pegs and kept the areas mulched with their local mulch and fertilized once I saw the new shoot commencing their seasonal cycle.
Failing the above notice that terrestrial orchids usually flower, then are followed by a period of dormancy, very similar to many other exotic and native epiphytic orchids. The biggest difference is that the majority’s period of growth and flowering tends to be in the cooler months of the year following the wet season not during the wet season, while the dormant period is in the warmer drier months.
They can be dormant for 6 to 8 months of the year and need to be kept reasonably dry during this period. Ideal shade house conditions would be 30mm to 50mm shade with a solid alsynite roof. Our Fern Nursery comprised of a shade cloth section with alternating 30mm and 50mm shade cloth and the dry area was 2 sheet of alsynite with 2 sheets of corrugated iron running in a north south direction. This gave all the plants shade that was of different intensities during the day. This situation is ideal during the dormancy period for all plants.
Watering is by far the most crucial part growing terrestrial orchids successfully. When in active growth the plants should be watered regularly so that the potting mix remains moist at all times. As the plants finish flowering and start to die back they will commence to develop the new tubers. Watering at this stage needs to be decreased so that the mix is almost completely dry. If the mix remains wet at this time the tubers have a good chance of rotting. From early January start to water lightly occasionally (if it rains water lightly and if it storms water the plants thoroughly.) As the new growth appears and accelerates increase the water accordingly. I will stress here that rain water is far more beneficial than tap water.
Light varies depending on the genus, Pterostylis species and Acianthus species prefer shadier conditions (40mm to 60mm) than the Caladenia species, Caleana species and Diuris species (20mm to 30mm) the aptly named Sun Orchids Thelymitra species (10mm to 20mm) (The name applies to the plants because the flowers open as the sun rises and close by night). Too often enthusiast treat the orchids like babies locking them up in too dense a shade and create to humid conditions which lead to fungal and mite problems. All these species resent stagnant humid conditions so place them in a position where they receive good light and air flow.
Fertilizing is necessay for optimum growth and organic fertilizers offer the best method. Organic fertilizer like the ones we recommend delivered on a monthly basis from the onset of the leaves to the demise of the flowers is the best method we have learned over the years.
Pests may not be wanted but they do appear from time to time. The usual suspects are Slugs and Snails which can do immense damage in a very short period of time. Start placing upturned half orange and mandarin skins around near the plants as you begin to water. These baits will attract them as soon as they appear and before they increase in numbers. Keep the benches dry by watering in the mornings or keep the plants suspended on mesh benches is also a good deterrent. Thrips and Red Spider can at times also cause problems. Good air flow and drier conditions is usually enough to keep them at bay. If the problem doesn’t abate then a light application of natural pyrethrum or Garlic usually does the trick.
Potting mixes are important so use a good mix. Use our recommended open dry mix for terrestrial orchids. Add a small teaspoon of 100mm natural blood and bone to the mix for each 250mm standard pot. Repotting should be carried out annually during the dormant period. Remove the plants from the pots and gently remove the old soil being careful not to damage the tubers. Fill the new pot with the new mix to about 30mm below the surface. Replace the tubers on the new mix. Cover the tubers with 5mm of mix. Cover the surface with 10mm to 15mm of fine broken forest litter. Some enthusiasts insist on using Cassuarina or Allocasuarina needles. If the latter is used the logic is that they are more likely to prevent fungal problems and are easier for the orchid’s leaves to penetrate.
Pot size will depend on the number of tubers you have. A 100mm pot will handle 5 to 7 tubers, while a 150mm pot can handle 10 tubers and a 200mm pot 20 tubers. (The best plantings and flowerings appear in the 200mm pots.)
Further comments from Members:
All information is included in good faith and has been thoroughly researched prior to printing. The website or the author does not warrant or guarantee the accuracy of any information on these pages, nor does the website or the author accept any responsibility for any loss arising from the use of the information found within. The views and opinions are strictly those of the author or those members who chose to actively participate in the contents herein.
“Hi reader, it seems you use The Bible of Botany a lot. That’s great as we have great pleasure in bringing it to you! It’s a little awkward for us to ask, but our first aim is to purchase land approximately 1,600 hectares to link several parcels of N.P. into one at The Pinnacles NSW Australia, but we need your help. We’re not salespeople. We’re amateur botanists who have dedicated over 30 years to saving the environment in a practical way. We depend on donations to reach our goal. If you donate just $5, the price of your coffee this Sunday, We can help to keep the planet alive in a real way and continue to bring you regular updates and features on Australian plants all in one Botanical Bible. Any support is greatly appreciated. Thank you.”
In the spirit of reconciliation we acknowledge the Bundjalung, Gumbaynggirr and Yaegl and all aboriginal nations throughout Australia and their connections to land, sea and community. We pay our respect to their Elders past, present and future for the pleasures we have gained.